Selenium Lessens Osteoarthritis by Protecting Articular Chondrocytes from Oxidative Damage through Nrf2 and NF-κB Pathways
- PMID: 38473759
- PMCID: PMC10931631
- DOI: 10.3390/ijms25052511
Selenium Lessens Osteoarthritis by Protecting Articular Chondrocytes from Oxidative Damage through Nrf2 and NF-κB Pathways
Abstract
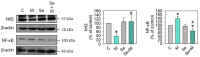
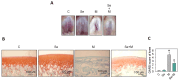
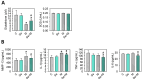
Osteoarthritis (OA) causes joint pain and disability due to the abnormal production of inflammatory cytokines and reactive oxygen species (ROS) in chondrocytes, leading to cell death and cartilage matrix destruction. Selenium (Se) intake can protect cells against oxidative damage. It is still unknown whether Se supplementation is beneficial for OA. This study investigated the effects of Se on sodium iodoacetate (MIA)-imitated OA progress in human chondrocyte cell line (SW1353 cells) and rats. The results showed that 0.3 μM of Se treatment could protect SW1353 cells from MIA-induced damage by the Nrf2 pathway by promoting the gene expression of glutathione-synthesis-related enzymes such as the glutamate-cysteine ligase catalytic subunit, the glutamate-cysteine ligase modifier subunit, and glutathione synthetase. In addition, glutathione, superoxide dismutase, glutathione peroxidase, and glutathione reductase expressions are also elevated to eliminate excessive ROS production. Moreover, Se could downregulate NF-κB, leading to a decrease in cytokines, matrix proteases, and glycosaminoglycans. In the rats, MIA-induced cartilage loss was lessened after 2 weeks of Se supplementation by oral gavage; meanwhile, glutathione synthesis was increased, and the expressions of pro-inflammatory cytokines were decreased. These results suggest that Se intake is beneficial for OA due to its effects of decreasing cartilage loss by enhancing antioxidant capacity and reducing inflammation.
Keywords: anti-inflammation; antioxidant; chondrocyte; minerals; osteoarthritis; selenium.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures





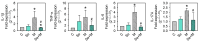

Similar articles
-
Selenium protects against Pb-induced renal oxidative injury in weaning rats and human renal tubular epithelial cells through activating NRF2.J Trace Elem Med Biol. 2024 May;83:127420. doi: 10.1016/j.jtemb.2024.127420. Epub 2024 Feb 27. J Trace Elem Med Biol. 2024. PMID: 38432121
-
Heat-Killed Lactobacillus delbrueckii subsp. lactis 557 Extracts Protect Chondrocytes from Osteoarthritis Damage by Reducing Inflammation: An In Vitro Study.Nutrients. 2024 Dec 23;16(24):4417. doi: 10.3390/nu16244417. Nutrients. 2024. PMID: 39771038 Free PMC article.
-
Moderate mechanical stress suppresses chondrocyte ferroptosis in osteoarthritis by regulating NF-κB p65/GPX4 signaling pathway.Sci Rep. 2024 Mar 1;14(1):5078. doi: 10.1038/s41598-024-55629-x. Sci Rep. 2024. PMID: 38429394 Free PMC article.
-
Reactive oxygen species, aging and articular cartilage homeostasis.Free Radic Biol Med. 2019 Feb 20;132:73-82. doi: 10.1016/j.freeradbiomed.2018.08.038. Epub 2018 Aug 31. Free Radic Biol Med. 2019. PMID: 30176344 Free PMC article. Review.
-
Redox and NF-κB signaling in osteoarthritis.Free Radic Biol Med. 2019 Feb 20;132:90-100. doi: 10.1016/j.freeradbiomed.2018.09.025. Epub 2018 Sep 17. Free Radic Biol Med. 2019. PMID: 30236789 Review.
Cited by
-
WTAP mediates IL-1β-induced chondrocyte injury by enhancing CA12 mRNA stability depending on m6A modification.J Orthop Surg Res. 2024 Dec 5;19(1):826. doi: 10.1186/s13018-024-05262-1. J Orthop Surg Res. 2024. PMID: 39639339 Free PMC article.
-
The relationship between the composite dietary antioxidant index and rheumatoid arthritis risk in American adults: the mediating role of BMI.Front Immunol. 2025 Apr 9;16:1560570. doi: 10.3389/fimmu.2025.1560570. eCollection 2025. Front Immunol. 2025. PMID: 40270960 Free PMC article.
-
Circulating Essential Trace Element and Mineral Levels in Female Patients with Knee or Concomitant Knee and Hip Osteoarthritis.Biol Trace Elem Res. 2025 Jun 9. doi: 10.1007/s12011-025-04696-w. Online ahead of print. Biol Trace Elem Res. 2025. PMID: 40490590
-
Guarana, Selenium, and L-Carnitine Supplementation Improves the Oxidative Profile but Fails to Reduce Tissue Damage in Rats with Osteoarthritis.Antioxidants (Basel). 2025 Jul 18;14(7):881. doi: 10.3390/antiox14070881. Antioxidants (Basel). 2025. PMID: 40722985 Free PMC article.
-
Elemental Influence: The Emerging Role of Zinc, Copper, and Selenium in Osteoarthritis.Nutrients. 2025 Jun 21;17(13):2069. doi: 10.3390/nu17132069. Nutrients. 2025. PMID: 40647177 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

