Inhibition of Insulin-Regulated Aminopeptidase by Imidazo [1,5-α]pyridines-Synthesis and Evaluation
- PMID: 38473764
- PMCID: PMC10931632
- DOI: 10.3390/ijms25052516
Inhibition of Insulin-Regulated Aminopeptidase by Imidazo [1,5-α]pyridines-Synthesis and Evaluation
Abstract
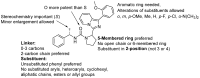
Inhibition of insulin-regulated aminopeptidase (IRAP) has been shown to improve cognitive functions in several animal models. Recently, we performed a screening campaign of approximately 10,000 compounds, identifying novel small-molecule-based compounds acting as inhibitors of the enzymatic activity of IRAP. Here we report on the chemical synthesis, structure-activity relationships (SAR) and initial characterization of physicochemical properties of a series of 48 imidazo [1,5-α]pyridine-based inhibitors, including delineation of their mode of action as non-competitive inhibitors with a small L-leucine-based IRAP substrate. The best compound displays an IC50 value of 1.0 µM. We elucidate the importance of two chiral sites in these molecules and find they have little impact on the compound's metabolic stability or physicochemical properties. The carbonyl group of a central urea moiety was initially believed to mimic substrate binding to a catalytically important Zn2+ ion in the active site, although the plausibility of this binding hypothesis is challenged by observation of excellent selectivity versus the closely related aminopeptidase N (APN). Taken together with the non-competitive inhibition pattern, we also consider an alternative model of allosteric binding.
Keywords: IRAP; enzyme inhibitor; imidazo [1,5-α]pyridine-based inhibitors; insulin-regulated aminopeptidase.
Conflict of interest statement
T.L. is currently an employee of AstraZeneca—all experimental work for this manuscript were performed while he was working for Chemical Biology Consortium Sweden.
Figures

Similar articles
-
Stabilization of the open conformation οf insulin-regulated aminopeptidase by a novel substrate-selective small-molecule inhibitor.Protein Sci. 2024 Sep;33(9):e5151. doi: 10.1002/pro.5151. Protein Sci. 2024. PMID: 39167040 Free PMC article.
-
The Discovery of New Inhibitors of Insulin-Regulated Aminopeptidase by a High-Throughput Screening of 400,000 Drug-like Compounds.Int J Mol Sci. 2024 Apr 6;25(7):4084. doi: 10.3390/ijms25074084. Int J Mol Sci. 2024. PMID: 38612894 Free PMC article.
-
Structural Basis of Inhibition of Human Insulin-Regulated Aminopeptidase (IRAP) by Benzopyran-Based Inhibitors.Front Mol Biosci. 2021 Apr 1;8:625274. doi: 10.3389/fmolb.2021.625274. eCollection 2021. Front Mol Biosci. 2021. PMID: 33869280 Free PMC article.
-
The Discovery of Insulin-Regulated Aminopeptidase (IRAP) Inhibitors: A Literature Review.Front Pharmacol. 2020 Sep 23;11:585838. doi: 10.3389/fphar.2020.585838. eCollection 2020. Front Pharmacol. 2020. PMID: 33071797 Free PMC article. Review.
-
Role of the insulin-regulated aminopeptidase IRAP in insulin action and diabetes.Biol Pharm Bull. 2004 Jun;27(6):761-4. doi: 10.1248/bpb.27.761. Biol Pharm Bull. 2004. PMID: 15187412 Review.
Cited by
-
Discovery of Novel Allosteric Inhibitor Hits for Insulin-Regulated Aminopeptidase Provides Insights on Enzymatic Mechanism.ACS Omega. 2025 Apr 23;10(17):17960-17972. doi: 10.1021/acsomega.5c01169. eCollection 2025 May 6. ACS Omega. 2025. PMID: 40352506 Free PMC article.
-
Quantum Mechanics/Molecular Mechanics Simulations Distinguish Insulin-Regulated Aminopeptidase Substrate (Oxytocin) and Inhibitor (Angiotensin IV) and Reveal Determinants of Activity and Inhibition.J Chem Inf Model. 2025 Jun 23;65(12):6261-6272. doi: 10.1021/acs.jcim.5c00869. Epub 2025 Jun 11. J Chem Inf Model. 2025. PMID: 40495656 Free PMC article.
-
Stabilization of the open conformation οf insulin-regulated aminopeptidase by a novel substrate-selective small-molecule inhibitor.Protein Sci. 2024 Sep;33(9):e5151. doi: 10.1002/pro.5151. Protein Sci. 2024. PMID: 39167040 Free PMC article.
-
The Discovery of New Inhibitors of Insulin-Regulated Aminopeptidase by a High-Throughput Screening of 400,000 Drug-like Compounds.Int J Mol Sci. 2024 Apr 6;25(7):4084. doi: 10.3390/ijms25074084. Int J Mol Sci. 2024. PMID: 38612894 Free PMC article.
References
-
- Wright J.W., Clemens J.A., Panetta J.A., Smalstig E.B., Weatherly L.A., Kramár E.A., Pederson E.S., Mungall B.H., Harding J.W. Effects of LY231617 and Angiotensin IV on Ischemia-Induced Deficits in Circular Water Maze and Passive Avoidance Performance in Rats. Brain Res. 1996;717:1–11. doi: 10.1016/0006-8993(95)01454-3. - DOI - PubMed
-
- Lee J., Albiston A.L., Allen A.M., Mendelsohn F.A.O., Ping S.E., Barrett G.L., Murphy M., Morris M.J., McDowall S.G., Chai S.Y. Effect of I.C.V. Injection of AT4 Receptor Ligands, NLE1-Angiotensin IV and LVV-Hemorphin 7, on Spatial Learning in Rats. Neuroscience. 2004;124:341–349. doi: 10.1016/j.neuroscience.2003.12.006. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

