Membrane-Driven Dimerization of the Peripheral Membrane Protein KRAS: Implications for Downstream Signaling
- PMID: 38473778
- PMCID: PMC10931714
- DOI: 10.3390/ijms25052530
Membrane-Driven Dimerization of the Peripheral Membrane Protein KRAS: Implications for Downstream Signaling
Abstract
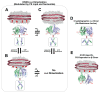
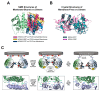
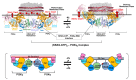
Transient homo-dimerization of the RAS GTPase at the plasma membrane has been shown to promote the mitogen-activated protein kinase (MAPK) signaling pathway essential for cell proliferation and oncogenesis. To date, numerous crystallographic studies have focused on the well-defined GTPase domains of RAS isoforms, which lack the disordered C-terminal membrane anchor, thus providing limited structural insight into membrane-bound RAS molecules. Recently, lipid-bilayer nanodisc platforms and paramagnetic relaxation enhancement (PRE) analyses have revealed several distinct structures of the membrane-anchored homodimers of KRAS, an isoform that is most frequently mutated in human cancers. The KRAS dimerization interface is highly plastic and altered by biologically relevant conditions, including oncogenic mutations, the nucleotide states of the protein, and the lipid composition. Notably, PRE-derived structures of KRAS homodimers on the membrane substantially differ in terms of the relative orientation of the protomers at an "α-α" dimer interface comprising two α4-α5 regions. This interface plasticity along with the altered orientations of KRAS on the membrane impact the accessibility of KRAS to downstream effectors and regulatory proteins. Further, nanodisc platforms used to drive KRAS dimerization can be used to screen potential anticancer drugs that target membrane-bound RAS dimers and probe their structural mechanism of action.
Keywords: KRAS; dimerization; nanodisc; paramagnetic relaxation enhancement (PRE); peripheral membrane protein.
Conflict of interest statement
The author declares no conflicts of interest.
Figures




Similar articles
-
Effector Binding Sequentially Alters KRAS Dimerization on the Membrane: New Insights Into RAS-Mediated RAF Activation.Adv Sci (Weinh). 2024 Oct;11(38):e2401530. doi: 10.1002/advs.202401530. Epub 2024 Aug 13. Adv Sci (Weinh). 2024. PMID: 39138901 Free PMC article.
-
Two Distinct Structures of Membrane-Associated Homodimers of GTP- and GDP-Bound KRAS4B Revealed by Paramagnetic Relaxation Enhancement.Angew Chem Int Ed Engl. 2020 Jun 26;59(27):11037-11045. doi: 10.1002/anie.202001758. Epub 2020 Apr 30. Angew Chem Int Ed Engl. 2020. PMID: 32227412 Free PMC article.
-
Conditional Cooperativity in RAS Assembly Pathways on Nanodiscs and Altered GTPase Cycling.Angew Chem Int Ed Engl. 2024 Mar 22;63(13):e202316942. doi: 10.1002/anie.202316942. Epub 2024 Feb 20. Angew Chem Int Ed Engl. 2024. PMID: 38305637
-
Is Nanoclustering essential for all oncogenic KRas pathways? Can it explain why wild-type KRas can inhibit its oncogenic variant?Semin Cancer Biol. 2019 Feb;54:114-120. doi: 10.1016/j.semcancer.2018.01.002. Epub 2018 Jan 5. Semin Cancer Biol. 2019. PMID: 29307569 Review.
-
Calmodulin and IQGAP1 activation of PI3Kα and Akt in KRAS, HRAS and NRAS-driven cancers.Biochim Biophys Acta Mol Basis Dis. 2018 Jun;1864(6 Pt B):2304-2314. doi: 10.1016/j.bbadis.2017.10.032. Epub 2017 Oct 31. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29097261 Review.
Cited by
-
KRas4b-Calmodulin Interaction with Membrane Surfaces: Role of Headgroup, Acyl Chain, and Electrostatics.Biochemistry. 2024 Nov 5;63(21):2740-2749. doi: 10.1021/acs.biochem.4c00116. Epub 2024 Oct 9. Biochemistry. 2024. PMID: 39382513
References
-
- Ahram M., Litou Z.I., Fang R., Al-Tawallbeh G. Estimation of membrane proteins in the human proteome. Silico Biol. 2006;6:379–386. - PubMed
-
- Cournia Z., Allen T.W., Andricioaei I., Antonny B., Baum D., Brannigan G., Buchete N.V., Deckman J.T., Delemotte L., del Val C., et al. Membrane Protein Structure, Function, and Dynamics: A Perspective from Experiments and Theory. J. Membr. Biol. 2015;248:611–640. doi: 10.1007/s00232-015-9802-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

