The Conjugates of Indolo[2,3- b]quinoline as Anti-Pancreatic Cancer Agents: Design, Synthesis, Molecular Docking and Biological Evaluations
- PMID: 38473820
- PMCID: PMC10932360
- DOI: 10.3390/ijms25052573
The Conjugates of Indolo[2,3- b]quinoline as Anti-Pancreatic Cancer Agents: Design, Synthesis, Molecular Docking and Biological Evaluations
Abstract
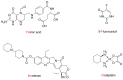
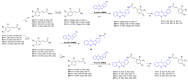
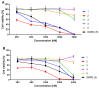
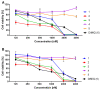
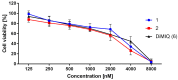
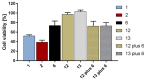
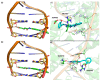
New amide conjugates of hydroxycinnamic acids (HCAs) and the known antineoplastic 5,11-dimethyl-5H-indolo[2,3-b]quinoline (DiMIQ), an analog of the natural alkaloid neocryptolepine, were synthesized and tested in vitro for anticancer activity. The compound 9-[((2-hydroxy)cinnamoyl)amino]-5,11-dimethyl-5H-indolo[2,3-b]quinoline (2), which contains the ortho-coumaric acid fragment, demonstrated dose-dependent effectiveness against both normal BxPC-3 and metastatic AsPC-1 pancreatic cancer cells. The IC50 values for AsPC-1 and BxPC-3 were 336.5 nM and 347.5 nM, respectively, with a selectivity index of approximately 5 for both pancreatic cancer cells compared to normal dermal fibroblasts. Conjugate 2 did not exhibit any hemolytic activity against human erythrocytes at the tested concentration. Computational studies were performed to predict the pharmacokinetic profile and potential mechanism of action of the synthesized conjugates. These studies focused on the ADME properties of the conjugates and their interactions with DNA, as well as DNA-topoisomerase alpha and beta complexes. All of the conjugates studied showed approximately one order of magnitude stronger binding to DNA compared to the reference DiMIQ, and approximately two orders of magnitude stronger binding to the topoisomerase II-DNA complex compared to DiMIQ. Conjugate 2 was predicted to have the strongest binding to the enzyme-DNA complex, with a Ki value of 2.8 nM.
Keywords: biological activity; heterocyclic moieties; molecular modeling; nature-derived molecules; organic synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
SYNTHESIS AND CYTOTOXIC ACTIVITY OF NEW 5H-INDOLO[2,3-B]QUINOLINE O-AMINOGLYCOSIDES.Acta Pol Pharm. 2016 May-Jun;73(3):683-92. Acta Pol Pharm. 2016. PMID: 27476287
-
Synthesis and structure-activity relationship of methyl-substituted indolo[2,3-b]quinolines: novel cytotoxic, DNA topoisomerase II inhibitors.J Med Chem. 1994 Oct 14;37(21):3503-10. doi: 10.1021/jm00047a008. J Med Chem. 1994. PMID: 7932579
-
Cytotoxicity and cell cycle effects of novel indolo[2,3-b]quinoline derivatives.Oncol Res. 2003;13(5):269-77. doi: 10.3727/096504003108748320. Oncol Res. 2003. PMID: 12688678
-
Sequence-selectivity of 5,11-dimethyl-5H-indolo[2,3-b]quinoline binding to DNA. Footprinting and molecular modeling studies.Bioorg Med Chem. 2000 May;8(5):937-43. doi: 10.1016/s0968-0896(00)00031-6. Bioorg Med Chem. 2000. PMID: 10882006
-
Identification of quinoline-chalcones and heterocyclic chalcone-appended quinolines as broad-spectrum pharmacological agents.Bioorg Chem. 2020 Dec;105:104419. doi: 10.1016/j.bioorg.2020.104419. Epub 2020 Oct 22. Bioorg Chem. 2020. PMID: 33142228 Review.
Cited by
-
The conjugates of 5'-deoxy-5-fluorocytidine and hydroxycinnamic acids - synthesis, anti-pancreatic cancer activity and molecular docking studies.RSC Adv. 2024 Apr 23;14(19):13129-13141. doi: 10.1039/d4ra01683a. eCollection 2024 Apr 22. RSC Adv. 2024. PMID: 38655481 Free PMC article.
-
Special Issue "Development and Synthesis of Biologically Active Compounds".Int J Mol Sci. 2024 Apr 4;25(7):4015. doi: 10.3390/ijms25074015. Int J Mol Sci. 2024. PMID: 38612824 Free PMC article.
References
-
- Klein-Brill A., Amar-Farkash S., Lawrence G., Collisson E.A., Aran D. Comparison of FOLFIRINOX vs Gemcitabine Plus Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Ductal Adenocarcinoma. JAMA Netw. Open. 2022;5:e2216199. doi: 10.1001/jamanetworkopen.2022.16199. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

