Dual Emissive Zn(II) Naphthalocyanines: Synthesis, Structural and Photophysical Characterization with Theory-Supported Insights towards Soluble Coordination Compounds with Visible and Near-Infrared Emission
- PMID: 38473852
- PMCID: PMC10931793
- DOI: 10.3390/ijms25052605
Dual Emissive Zn(II) Naphthalocyanines: Synthesis, Structural and Photophysical Characterization with Theory-Supported Insights towards Soluble Coordination Compounds with Visible and Near-Infrared Emission
Abstract
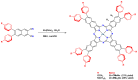
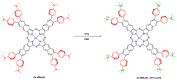
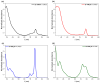
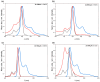
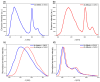
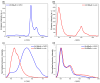
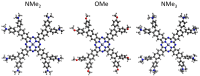
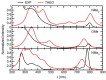
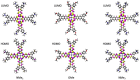
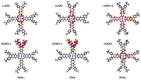
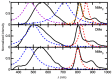
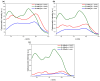
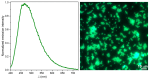
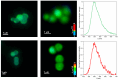
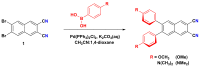
Metal phthalocyaninates and their higher homologues are recognized as deep-red luminophores emitting from their lowest excited singlet state. Herein, we report on the design, synthesis, and in-depth characterization of a new class of dual-emissive (visible and NIR) metal naphthalocyaninates. A 4-N,N-dimethylaminophen-4-yl-substituted naphthalocyaninato zinc(II) complex (Zn-NMe2Nc) and the derived water-soluble coordination compound (Zn-NMe3Nc) exhibit a near-infrared fluorescence from the lowest ligand-centered state, along with a unique push-pull-supported luminescence in the visible region of the electromagnetic spectrum. An unprecedentedly broad structural (2D-NMR spectroscopy and mass spectrometry) as well as photophysical characterization (steady-state state and time-resolved photoluminescence spectroscopy) is presented. The unique dual emission was assigned to two independent sets of singlet states related to the intrinsic Q-band of the macrocycle and to the push-pull substituents in the molecular periphery, respectively, as predicted by TD-DFT calculations. In general, the elusive chemical aspects of these macrocyclic compounds are addressed, involving both reaction conditions, thorough purification, and in-depth characterization. Besides the fundamental aspects that are investigated herein, the photoacoustic properties were exemplarily examined using phantom gels to assess their tomographic imaging capabilities. Finally, the robust luminescence in the visible range arising from the push-pull character of the peripheral moieties demonstrated a notable independence from aggregation and was exemplarily implemented for optical imaging (FLIM) through time-resolved multiphoton micro(spectro)scopy.
Keywords: (TD-)DFT; dual fluorescence; multiscale–multimodal imaging; photoacoustic/optoacoustic spectroscopy; steady-state and time-resolved multiphoton micro(spectro)scopy (FLIM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sung R., Sung K. TICT Excited States of o- and p-N,N-Dimethylamino Analogues of GFP Chromophore. J. Phys. Org. Chem. 2018;31:e3791. doi: 10.1002/poc.3791. - DOI
-
- Lippert E., Lüder W., Moll F., Nägele W., Boos H., Prigge H., Seibold-Blankenstein I. Umwandlung von Elektronenanregungsenergie. Angew. Chem. 1961;73:695–706. doi: 10.1002/ange.19610732103. - DOI
-
- Chou P.-T., Pu S.-C., Cheng Y.-M., Yu W.-S., Yu Y.-C., Hung F.-T., Hu W.-P. Femtosecond Dynamics on Excited-State Proton/Charge-Transfer Reaction in 4’-N,N-Diethylamino-3-Hydroxyflavone. The Role of Dipolar Vectors in Constructing a Rational Mechanism. J. Phys. Chem. A. 2005;109:3777–3787. doi: 10.1021/jp044205w. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- CRC 1450 - 431460824 (projects A02 and A03)/Deutsche Forschungsgemeinschaft
- INST 211/915-1 FUGG: "Integrated confocal luminescence spectrometer with spatiotemporal resolution and multiphoton excitation"/DFG
- EXC 1003 Cluster of Excellence Cells in Motion (Berufungsmittel)/Deutsche Forschungsgemeinschaft
- Doctoral fellowship for STNS/German Academic Exchange Service
- MSOT/Interdisciplinary Center for Clinical Research (IZKF core unit PIX), Münster
LinkOut - more resources
Full Text Sources
Miscellaneous

