Oxytocin Modifies the Excitability and the Action Potential Shape of the Hippocampal CA1 GABAergic Interneurons
- PMID: 38473860
- PMCID: PMC10932319
- DOI: 10.3390/ijms25052613
Oxytocin Modifies the Excitability and the Action Potential Shape of the Hippocampal CA1 GABAergic Interneurons
Abstract
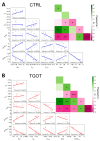
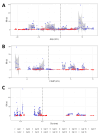
Oxytocin (OT) is a neuropeptide that modulates social-related behavior and cognition in the central nervous system of mammals. In the CA1 area of the hippocampus, the indirect effects of the OT on the pyramidal neurons and their role in information processing have been elucidated. However, limited data are available concerning the direct modulation exerted by OT on the CA1 interneurons (INs) expressing the oxytocin receptor (OTR). Here, we demonstrated that TGOT (Thr4,Gly7-oxytocin), a selective OTR agonist, affects not only the membrane potential and the firing frequency but also the neuronal excitability and the shape of the action potentials (APs) of these INs in mice. Furthermore, we constructed linear mixed-effects models (LMMs) to unravel the dependencies between the AP parameters and the firing frequency, also considering how TGOT can interact with them to strengthen or weaken these influences. Our analyses indicate that OT regulates the functionality of the CA1 GABAergic INs through different and independent mechanisms. Specifically, the increase in neuronal firing rate can be attributed to the depolarizing effect on the membrane potential and the related enhancement in cellular excitability by the peptide. In contrast, the significant changes in the AP shape are directly linked to oxytocinergic modulation. Importantly, these alterations in AP shape are not associated with the TGOT-induced increase in neuronal firing rate, being themselves critical for signal processing.
Keywords: CA1; GABAergic interneurons; electrophysiology; hippocampus; linear mixed-effects models; oxytocin; patch-clamp; phase plot analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale.Eur J Neurosci. 2000 Nov;12(11):3975-84. doi: 10.1046/j.1460-9568.2000.00290.x. Eur J Neurosci. 2000. PMID: 11069593
-
Oxytocin Increases Phasic and Tonic GABAergic Transmission in CA1 Region of Mouse Hippocampus.Front Cell Neurosci. 2019 May 7;13:178. doi: 10.3389/fncel.2019.00178. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31133808 Free PMC article.
-
Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons of the rat spinal cord.Mol Pain. 2009 Nov 12;5:63. doi: 10.1186/1744-8069-5-63. Mol Pain. 2009. PMID: 19909537 Free PMC article.
-
Oxytocin excites BNST interneurons and inhibits BNST output neurons to the central amygdala.Neuropharmacology. 2021 Jul 1;192:108601. doi: 10.1016/j.neuropharm.2021.108601. Epub 2021 May 7. Neuropharmacology. 2021. PMID: 33971215 Free PMC article.
-
Neuromodulatory functions exerted by oxytocin on different populations of hippocampal neurons in rodents.Front Cell Neurosci. 2023 Feb 2;17:1082010. doi: 10.3389/fncel.2023.1082010. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36816855 Free PMC article. Review.
Cited by
-
Dual Oxytocin Signals in Striatal Astrocytes.Biomolecules. 2025 Aug 4;15(8):1122. doi: 10.3390/biom15081122. Biomolecules. 2025. PMID: 40867567 Free PMC article.
-
Oxytocin-Mediate Modulation of Splenic Immunosuppression in Chronic Social Stress Through Neuroendocrine Pathways.Adv Sci (Weinh). 2025 Jul;12(25):e2500849. doi: 10.1002/advs.202500849. Epub 2025 Apr 26. Adv Sci (Weinh). 2025. PMID: 40285614 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

