Mechanism of Action of Antitumor Au(I) N-Heterocyclic Carbene Complexes: A Computational Insight on the Targeting of TrxR Selenocysteine
- PMID: 38473872
- PMCID: PMC10932012
- DOI: 10.3390/ijms25052625
Mechanism of Action of Antitumor Au(I) N-Heterocyclic Carbene Complexes: A Computational Insight on the Targeting of TrxR Selenocysteine
Abstract
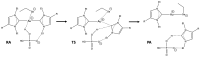
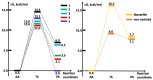
The targeting of human thioredoxin reductase is widely recognized to be crucially involved in the anticancer properties of several metallodrugs, including Au(I) complexes. In this study, the mechanism of reaction between a set of five N-heterocyclic carbene Au(I) complexes and models of the active Sec residue in human thioredoxin reductase was investigated by means of density functional theory approaches. The study was specifically addressed to the kinetics and thermodynamics of the tiled process by aiming at elucidating and explaining the differential inhibitory potency in this set of analogous Au(I) bis-carbene complexes. While the calculated free energy profile showed a substantially similar reactivity, we found that the binding of these Au(I) bis-carbene at the active CysSec dyad in the TrxR enzyme could be subjected to steric and orientational restraints, underlining both the approach of the bis-carbene scaffold and the attack of the selenol group at the metal center. A new and detailed mechanistic insight to the anticancer activity of these Au(I) organometallic complexes was thus provided by consolidating the TrxR targeting paradigm.
Keywords: DFT calculations; N-heterocyclic carbenes; anticancer metallodrugs; gold(I) complexes; proteins; thioredoxin reductase TrxR.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Characterization of Hydrophilic Gold(I) N-Heterocyclic Carbene (NHC) Complexes as Potent TrxR Inhibitors Using Biochemical and Mass Spectrometric Approaches.Inorg Chem. 2017 Nov 20;56(22):14237-14250. doi: 10.1021/acs.inorgchem.7b02345. Epub 2017 Nov 2. Inorg Chem. 2017. PMID: 29095609
-
Gold(I) NHC Complexes: Antiproliferative Activity, Cellular Uptake, Inhibition of Mammalian and Bacterial Thioredoxin Reductases, and Gram-Positive Directed Antibacterial Effects.Chemistry. 2017 Feb 3;23(8):1869-1880. doi: 10.1002/chem.201604512. Epub 2017 Jan 10. Chemistry. 2017. PMID: 27865002
-
Reactivity of antitumor coinage metal-based N-heterocyclic carbene complexes with cysteine and selenocysteine protein sites.J Inorg Biochem. 2021 Oct;223:111533. doi: 10.1016/j.jinorgbio.2021.111533. Epub 2021 Jul 9. J Inorg Biochem. 2021. PMID: 34273714
-
Chemistry, structure, and biological roles of Au-NHC complexes as TrxR inhibitors.Bioorg Chem. 2020 Jan;95:103552. doi: 10.1016/j.bioorg.2019.103552. Epub 2019 Dec 24. Bioorg Chem. 2020. PMID: 31911299 Review.
-
Gold-NHC complexes: from synthetic aspects to anti-cancer activity.Dalton Trans. 2025 May 13;54(19):7553-7601. doi: 10.1039/d5dt00118h. Dalton Trans. 2025. PMID: 40171803 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

