Targeted Suicide Gene Therapy with Retroviral Replicating Vectors for Experimental Canine Cancers
- PMID: 38473904
- PMCID: PMC10932112
- DOI: 10.3390/ijms25052657
Targeted Suicide Gene Therapy with Retroviral Replicating Vectors for Experimental Canine Cancers
Abstract
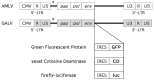
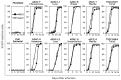
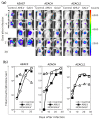
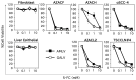
Cancer in dogs has increased in recent years and is a leading cause of death. We have developed a retroviral replicating vector (RRV) that specifically targets cancer cells for infection and replication. RRV carrying a suicide gene induced synchronized killing of cancer cells when administered with a prodrug after infection. In this study, we evaluated two distinct RRVs derived from amphotropic murine leukemia virus (AMLV) and gibbon ape leukemia virus (GALV) in canine tumor models both in vitro and in vivo. Despite low infection rates in normal canine cells, both RRVs efficiently infected and replicated within all the canine tumor cells tested. The efficient intratumoral spread of the RRVs after their intratumoral injection was also demonstrated in nude mouse models of subcutaneous canine tumor xenografts. When both RRVs encoded a yeast cytosine deaminase suicide gene, which converts the prodrug 5-fluorocytosine (5-FC) to the active drug 5-fluorouracil, they caused tumor-cell-specific 5-FC-induced killing of the canine tumor cells in vitro. Furthermore, in the AZACF- and AZACH-cell subcutaneous tumor xenograft models, both RRVs exerted significant antitumor effects. These results suggest that RRV-mediated suicide gene therapy is a novel therapeutic approach to canine cancers.
Keywords: canine cancer; retroviral replicating vectors; suicide gene therapy.
Conflict of interest statement
N.K. is a former consultant of Tocagen Inc. This does not alter the authors’ adherence to all MDPI policies on sharing data and materials. The other authors declare no conflicts of interest.
Figures

Similar articles
-
Highly efficient tumor transduction and antitumor efficacy in experimental human malignant mesothelioma using replicating gibbon ape leukemia virus.Cancer Gene Ther. 2013 Dec;20(12):671-7. doi: 10.1038/cgt.2013.67. Epub 2013 Nov 8. Cancer Gene Ther. 2013. PMID: 24201868 Free PMC article.
-
Therapeutic Efficacy of Prodrug Activator Gene Therapy Using Retroviral Replicating Vectors for Human Ovarian Cancer.Anticancer Res. 2023 Dec;43(12):5311-5317. doi: 10.21873/anticanres.16734. Anticancer Res. 2023. PMID: 38030176
-
Retroviral Replicating Vectors Mediated Prodrug Activator Gene Therapy in a Gastric Cancer Model.Int J Mol Sci. 2023 Oct 2;24(19):14823. doi: 10.3390/ijms241914823. Int J Mol Sci. 2023. PMID: 37834271 Free PMC article.
-
Replicating retroviral vectors for oncolytic virotherapy of experimental hepatocellular carcinoma.Oncol Rep. 2012 Jul;28(1):21-6. doi: 10.3892/or.2012.1789. Epub 2012 Apr 26. Oncol Rep. 2012. PMID: 22552490
-
Clinical development of retroviral replicating vector Toca 511 for gene therapy of cancer.Expert Opin Biol Ther. 2021 Sep;21(9):1199-1214. doi: 10.1080/14712598.2021.1902982. Epub 2021 May 6. Expert Opin Biol Ther. 2021. PMID: 33724117 Free PMC article. Review.
References
-
- Mizuno T. Spontaneously occurring canine cancer as a relevant animal model for developing novel treatments for human cancers. Transl. Regul. Sci. 2021;3:51–59. doi: 10.33611/trs.2021-007. - DOI
-
- Volovat S.R., Scripcariu D.V., Vasilache I.A., Stolniceanu C.R., Volovat C., Augustin I.G., Volovat C.C., Ostafe M.R., Andreea-Voichița S.G., Bejusca-Vieriu T., et al. Oncolytic Virotherapy: A New Paradigm in Cancer Immunotherapy. Int. J. Mol. Sci. 2024;25:1180. doi: 10.3390/ijms25021180. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

