Novel Perspectives in Chronic Kidney Disease-Specific Cardiovascular Disease
- PMID: 38473905
- PMCID: PMC10931927
- DOI: 10.3390/ijms25052658
Novel Perspectives in Chronic Kidney Disease-Specific Cardiovascular Disease
Abstract
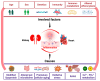
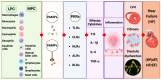
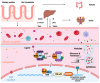
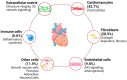
Chronic kidney disease (CKD) affects > 10% of the global adult population and significantly increases the risk of cardiovascular disease (CVD), which remains the leading cause of death in this population. The development and progression of CVD-compared to the general population-is premature and accelerated, manifesting as coronary artery disease, heart failure, arrhythmias, and sudden cardiac death. CKD and CV disease combine to cause multimorbid cardiorenal syndrome (CRS) due to contributions from shared risk factors, including systolic hypertension, diabetes mellitus, obesity, and dyslipidemia. Additional neurohormonal activation, innate immunity, and inflammation contribute to progressive cardiac and renal deterioration, reflecting the strong bidirectional interaction between these organ systems. A shared molecular pathophysiology-including inflammation, oxidative stress, senescence, and hemodynamic fluctuations characterise all types of CRS. This review highlights the evolving paradigm and recent advances in our understanding of the molecular biology of CRS, outlining the potential for disease-specific therapies and biomarker disease detection.
Keywords: Chronic kidney disease; biomarkers; cardiorenal syndrome; cardiovascular disease; inflammation.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures
References
-
- Xie Y., Bowe B., Mokdad A.H., Xian H., Yan Y., Li T., Maddukuri G., Tsai C.Y., Floyd T., Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

