Osteoimmunology: The Crosstalk between T Cells, B Cells, and Osteoclasts in Rheumatoid Arthritis
- PMID: 38473934
- PMCID: PMC10931770
- DOI: 10.3390/ijms25052688
Osteoimmunology: The Crosstalk between T Cells, B Cells, and Osteoclasts in Rheumatoid Arthritis
Abstract
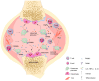
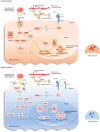
Rheumatoid arthritis (RA) is an ongoing inflammatory condition that affects the joints and can lead to severe damage to cartilage and bones, resulting in significant disability. This condition occurs when the immune system becomes overactive, causing osteoclasts, cells responsible for breaking down bone, to become more active than necessary, leading to bone breakdown. RA disrupts the equilibrium between osteoclasts and osteoblasts, resulting in serious complications such as localized bone erosion, weakened bones surrounding the joints, and even widespread osteoporosis. Antibodies against the receptor activator of nuclear factor-κB ligand (RANKL), a crucial stimulator of osteoclast differentiation, have shown great effectiveness both in laboratory settings and actual patient cases. Researchers are increasingly focusing on osteoclasts as significant contributors to bone erosion in RA. Given that RA involves an overactive immune system, T cells and B cells play a pivotal role by intensifying the immune response. The imbalance between Th17 cells and Treg cells, premature aging of T cells, and excessive production of antibodies by B cells not only exacerbate inflammation but also accelerate bone destruction. Understanding the connection between the immune system and osteoclasts is crucial for comprehending the impact of RA on bone health. By delving into the immune mechanisms that lead to joint damage, exploring the interactions between the immune system and osteoclasts, and investigating new biomarkers for RA, we can significantly improve early diagnosis, treatment, and prognosis of this condition.
Keywords: B cell; T cell; osteoclasts; osteoimmunology; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Rheumatoid arthritis and osteoimmunology: The adverse impact of a deregulated immune system on bone metabolism.Bone. 2022 Sep;162:116468. doi: 10.1016/j.bone.2022.116468. Epub 2022 Jun 8. Bone. 2022. PMID: 35688359 Review.
-
Clinical immunity in bone and joints.J Bone Miner Metab. 2019 Jan;37(1):2-8. doi: 10.1007/s00774-018-0965-5. Epub 2018 Oct 15. J Bone Miner Metab. 2019. PMID: 30324535 Review.
-
Immune-bone interplay in the structural damage in rheumatoid arthritis.Clin Exp Immunol. 2018 Oct;194(1):1-8. doi: 10.1111/cei.13188. Epub 2018 Sep 4. Clin Exp Immunol. 2018. PMID: 30022480 Free PMC article. Review.
-
Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review.JBI Libr Syst Rev. 2012;10(42 Suppl):1-12. doi: 10.11124/jbisrir-2012-288. JBI Libr Syst Rev. 2012. PMID: 27820156
-
Cytokine-mediated bone destruction in rheumatoid arthritis.J Immunol Res. 2014;2014:263625. doi: 10.1155/2014/263625. Epub 2014 Sep 10. J Immunol Res. 2014. PMID: 25295284 Free PMC article. Review.
Cited by
-
Unraveling the causal role of TGF-βRII in osteoporosis and the potential of its associated differential genes as novel targets.Eur J Med Res. 2025 Aug 18;30(1):756. doi: 10.1186/s40001-025-02971-z. Eur J Med Res. 2025. PMID: 40826484
-
Synergistic roles of NFATc1 and c-Jun in immunomodulation.Biochem Biophys Rep. 2025 Jul 7;43:102137. doi: 10.1016/j.bbrep.2025.102137. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40688507 Free PMC article. Review.
-
Animal Models in Rheumatoid Arthritis: Is There a Correlation Between Autoantibodies in Human Pathology and Animal Models?Biology (Basel). 2025 Apr 24;14(5):460. doi: 10.3390/biology14050460. Biology (Basel). 2025. PMID: 40427650 Free PMC article. Review.
-
A new 3-arylbenzofuran derivative EIE-2 reestablishes Treg-dependent tolerance in rheumatoid arthritis by targeting on Syk induced mTOR and PKCθ imbalance.Front Immunol. 2025 May 21;16:1524491. doi: 10.3389/fimmu.2025.1524491. eCollection 2025. Front Immunol. 2025. PMID: 40469311 Free PMC article.
-
Rheumatoid Arthritis Aggravates Disease Severity and the Risk of Postoperative Recurrence in Chronic Rhinosinusitis.J Inflamm Res. 2025 Jul 16;18:9401-9411. doi: 10.2147/JIR.S533652. eCollection 2025. J Inflamm Res. 2025. PMID: 40692544 Free PMC article.
References
-
- Maeda K., Yoshida K., Nishizawa T., Otani K., Yamashita Y., Okabe H., Hadano Y., Kayama T., Kurosaka D., Saito M. Inflammation and Bone Metabolism in Rheumatoid Arthritis: Molecular Mechanisms of Joint Destruction and Pharmacological Treatments. Int. J. Mol. Sci. 2022;23:2871. doi: 10.3390/ijms23052871. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

