Functional Studies of Deafness-Associated Pendrin and Prestin Variants
- PMID: 38474007
- PMCID: PMC10931795
- DOI: 10.3390/ijms25052759
Functional Studies of Deafness-Associated Pendrin and Prestin Variants
Abstract
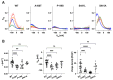
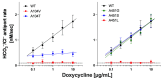
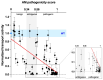
Pendrin and prestin are evolutionary-conserved membrane proteins that are essential for normal hearing. Dysfunction of these proteins results in hearing loss in humans, and numerous deafness-associated pendrin and prestin variants have been identified in patients. However, the pathogenic impacts of many of these variants are ambiguous. Here, we report results from our ongoing efforts to experimentally characterize pendrin and prestin variants using in vitro functional assays. With previously established fluorometric anion transport assays, we determined that many of the pendrin variants identified on transmembrane (TM) 10, which contains the essential anion binding site, and on the neighboring TM9 within the core domain resulted in impaired anion transport activity. We also determined the range of functional impairment in three deafness-associated prestin variants by measuring nonlinear capacitance (NLC), a proxy for motor function. Using the results from our functional analyses, we also evaluated the performance of AlphaMissense (AM), a computational tool for predicting the pathogenicity of missense variants. AM prediction scores correlated well with our experimental results; however, some variants were misclassified, underscoring the necessity of experimentally assessing the effects of variants. Together, our experimental efforts provide invaluable information regarding the pathogenicity of deafness-associated pendrin and prestin variants.
Keywords: DFNB4; DFNB61; SLC26A4; SLC26A5; hereditary hearing loss; nonlinear capacitance; pendred syndrome; pendrin; prestin.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures



Update of
-
Functional studies of deafness-associated pendrin and prestin variants.bioRxiv [Preprint]. 2024 Jan 26:2024.01.23.576877. doi: 10.1101/2024.01.23.576877. bioRxiv. 2024. Update in: Int J Mol Sci. 2024 Feb 27;25(5):2759. doi: 10.3390/ijms25052759. PMID: 38328051 Free PMC article. Updated. Preprint.
References
-
- Choi B.Y., Kim H.M., Ito T., Lee K.Y., Li X., Monahan K., Wen Y., Wilson E., Kurima K., Saunders T.L., et al. Mouse model of enlarged vestibular aqueducts defines temporal requirement of Slc26a4 expression for hearing acquisition. J. Clin. Investig. 2011;121:4516–4525. doi: 10.1172/JCI59353. - DOI - PMC - PubMed
-
- Everett L.A., Belyantseva I.A., Noben-Trauth K., Cantos R., Chen A., Thakkar S.I., Hoogstraten-Miller S.L., Kachar B., Wu D.K., Green E.D. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum. Mol. Genet. 2001;10:153–161. doi: 10.1093/hmg/10.2.153. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

