Matrix Metalloproteinases in the Periodontium-Vital in Tissue Turnover and Unfortunate in Periodontitis
- PMID: 38474009
- PMCID: PMC10932118
- DOI: 10.3390/ijms25052763
Matrix Metalloproteinases in the Periodontium-Vital in Tissue Turnover and Unfortunate in Periodontitis
Abstract
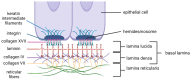
The extracellular matrix (ECM) is a complex non-cellular three-dimensional macromolecular network present within all tissues and organs, forming the foundation on which cells sit, and composed of proteins (such as collagen), glycosaminoglycans, proteoglycans, minerals, and water. The ECM provides a fundamental framework for the cellular constituents of tissue and biochemical support to surrounding cells. The ECM is a highly dynamic structure that is constantly being remodeled. Matrix metalloproteinases (MMPs) are among the most important proteolytic enzymes of the ECM and are capable of degrading all ECM molecules. MMPs play a relevant role in physiological as well as pathological processes; MMPs participate in embryogenesis, morphogenesis, wound healing, and tissue remodeling, and therefore, their impaired activity may result in several problems. MMP activity is also associated with chronic inflammation, tissue breakdown, fibrosis, and cancer invasion and metastasis. The periodontium is a unique anatomical site, composed of a variety of connective tissues, created by the ECM. During periodontitis, a chronic inflammation affecting the periodontium, increased presence and activity of MMPs is observed, resulting in irreversible losses of periodontal tissues. MMP expression and activity may be controlled in various ways, one of which is the inhibition of their activity by an endogenous group of tissue inhibitors of metalloproteinases (TIMPs), as well as reversion-inducing cysteine-rich protein with Kazal motifs (RECK).
Keywords: extracellular matrix; matrix metalloproteinases; periodontal diseases; periodontitis.
Conflict of interest statement
Authors declare no conflict of interest.
Figures


Similar articles
-
Potential Role of Reversion-Inducing Cysteine-Rich Protein with Kazal Motifs (RECK) in Regulation of Matrix Metalloproteinases (MMPs) Expression in Periodontal Diseases.Med Sci Monit. 2016 Jun 7;22:1936-8. doi: 10.12659/msm.896546. Med Sci Monit. 2016. PMID: 27272560 Free PMC article.
-
Expression of matrix metalloproteinases-2, -9 and reversion-inducing cysteine-rich protein with Kazal motifs in gingiva in periodontal health and disease.Arch Oral Biol. 2017 Mar;75:62-67. doi: 10.1016/j.archoralbio.2016.12.009. Epub 2016 Dec 26. Arch Oral Biol. 2017. PMID: 28043014
-
Is collagen breakdown during periodontitis linked to inflammatory cells and expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival tissue?J Periodontol. 2001 Oct;72(10):1398-406. doi: 10.1902/jop.2001.72.10.1398. J Periodontol. 2001. PMID: 11699482
-
RECK, a novel matrix metalloproteinase regulator.Histol Histopathol. 2008 Aug;23(8):1003-10. doi: 10.14670/HH-23.1003. Histol Histopathol. 2008. PMID: 18498076 Review.
-
Turnover in periodontal connective tissues: dynamic homeostasis of cells, collagen and ground substances.Oral Dis. 1995 Dec;1(4):238-53. doi: 10.1111/j.1601-0825.1995.tb00189.x. Oral Dis. 1995. PMID: 8705832 Review.
Cited by
-
Nutritional Benefits of Lycopene and Beta-Carotene: A Comprehensive Overview.Food Sci Nutr. 2024 Oct 16;12(11):8715-8741. doi: 10.1002/fsn3.4502. eCollection 2024 Nov. Food Sci Nutr. 2024. PMID: 39619948 Free PMC article. Review.
-
Impact of Aging and Pathologies on Human Oral Mucosa: Preliminary Investigation of Biophysical Markers from Thermal and Vibrational Analyses.Biomolecules. 2025 Jul 8;15(7):978. doi: 10.3390/biom15070978. Biomolecules. 2025. PMID: 40723850 Free PMC article.
-
Matrix Dynamics and Microbiome Crosstalk: Matrix Metalloproteinases as Key Players in Disease and Therapy.Int J Mol Sci. 2025 Apr 11;26(8):3621. doi: 10.3390/ijms26083621. Int J Mol Sci. 2025. PMID: 40332093 Free PMC article. Review.
-
Integrated machine learning and single-cell RNA sequencing reveal COL4A2 and CXCL6 as oxidative stress-associated biomarkers in periodontitis.Front Immunol. 2025 Jun 5;16:1598642. doi: 10.3389/fimmu.2025.1598642. eCollection 2025. Front Immunol. 2025. PMID: 40539067 Free PMC article.
-
New Insights in Natural Bioactive Compounds for Periodontal Disease: Advanced Molecular Mechanisms and Therapeutic Potential.Molecules. 2025 Feb 10;30(4):807. doi: 10.3390/molecules30040807. Molecules. 2025. PMID: 40005119 Free PMC article. Review.
References
-
- Słotwińska S.M. The Immunologic Aspects of Periodontal Disease. Cent. Eur. J. Immunol. 2011;36:279–283.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

