Targeting ATR Pathway in Solid Tumors: Evidence of Improving Therapeutic Outcomes
- PMID: 38474014
- PMCID: PMC10932434
- DOI: 10.3390/ijms25052767
Targeting ATR Pathway in Solid Tumors: Evidence of Improving Therapeutic Outcomes
Abstract
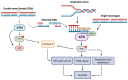
The DNA damage response (DDR) system is a complicated network of signaling pathways that detects and repairs DNA damage or induces apoptosis. Critical regulators of the DDR network include the DNA damage kinases ataxia telangiectasia mutated Rad3-related kinase (ATR) and ataxia-telangiectasia mutated (ATM). The ATR pathway coordinates processes such as replication stress response, stabilization of replication forks, cell cycle arrest, and DNA repair. ATR inhibition disrupts these functions, causing a reduction of DNA repair, accumulation of DNA damage, replication fork collapse, inappropriate mitotic entry, and mitotic catastrophe. Recent data have shown that the inhibition of ATR can lead to synthetic lethality in ATM-deficient malignancies. In addition, ATR inhibition plays a significant role in the activation of the immune system by increasing the tumor mutational burden and neoantigen load as well as by triggering the accumulation of cytosolic DNA and subsequently inducing the cGAS-STING pathway and the type I IFN response. Taken together, we review stimulating data showing that ATR kinase inhibition can alter the DDR network, the immune system, and their interplay and, therefore, potentially provide a novel strategy to improve the efficacy of antitumor therapy, using ATR inhibitors as monotherapy or in combination with genotoxic drugs and/or immunomodulators.
Keywords: ATR; ATR inhibitor; ATR-ATM interplay; DNA damage response; ceralasertib (AZD6738); immune system; synthetic lethality.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Cancer Vulnerabilities Through Targeting the ATR/Chk1 and ATM/Chk2 Axes in the Context of DNA Damage.Cells. 2025 May 20;14(10):748. doi: 10.3390/cells14100748. Cells. 2025. PMID: 40422251 Free PMC article. Review.
-
ATR inhibition induces synthetic lethality and overcomes chemoresistance in TP53- or ATM-defective chronic lymphocytic leukemia cells.Blood. 2016 Feb 4;127(5):582-95. doi: 10.1182/blood-2015-05-644872. Epub 2015 Nov 12. Blood. 2016. PMID: 26563132
-
Therapeutic Targeting of the DNA Damage Response Using an ATR Inhibitor in Biliary Tract Cancer.Cancer Res Treat. 2019 Jul;51(3):1167-1179. doi: 10.4143/crt.2018.526. Epub 2018 Dec 3. Cancer Res Treat. 2019. PMID: 30514066 Free PMC article.
-
Depletion of ATR selectively sensitizes ATM-deficient human mammary epithelial cells to ionizing radiation and DNA-damaging agents.Cell Cycle. 2014;13(22):3541-50. doi: 10.4161/15384101.2014.960729. Cell Cycle. 2014. PMID: 25483091 Free PMC article.
-
Targeting ATM and ATR for cancer therapeutics: Inhibitors in clinic.Drug Discov Today. 2023 Aug;28(8):103662. doi: 10.1016/j.drudis.2023.103662. Epub 2023 Jun 10. Drug Discov Today. 2023. PMID: 37302542 Review.
Cited by
-
The Interplay between the DNA Damage Response (DDR) Network and the Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway in Multiple Myeloma.Int J Mol Sci. 2024 Jun 26;25(13):6991. doi: 10.3390/ijms25136991. Int J Mol Sci. 2024. PMID: 39000097 Free PMC article. Review.
-
Elucidating DNA Damage-Dependent Immune System Activation.Int J Mol Sci. 2025 Jun 18;26(12):5849. doi: 10.3390/ijms26125849. Int J Mol Sci. 2025. PMID: 40565309 Free PMC article. Review.
-
Discovery of novel serum peptide biomarkers for cholangiocarcinoma recurrence through MALDI-TOF MS and LC-MS/MS peptidome analysis.Sci Rep. 2025 Jan 20;15(1):2582. doi: 10.1038/s41598-025-87124-2. Sci Rep. 2025. PMID: 39833435 Free PMC article.
-
Identification of PRMT5 as a therapeutic target in cholangiocarcinoma.Gut. 2024 Dec 10;74(1):116-127. doi: 10.1136/gutjnl-2024-332998. Gut. 2024. PMID: 39266051 Free PMC article.
-
Cancer Vulnerabilities Through Targeting the ATR/Chk1 and ATM/Chk2 Axes in the Context of DNA Damage.Cells. 2025 May 20;14(10):748. doi: 10.3390/cells14100748. Cells. 2025. PMID: 40422251 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous