Comprehensive Assessment of CFTR Modulators' Therapeutic Efficiency for N1303K Variant
- PMID: 38474016
- PMCID: PMC10931982
- DOI: 10.3390/ijms25052770
Comprehensive Assessment of CFTR Modulators' Therapeutic Efficiency for N1303K Variant
Abstract
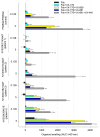
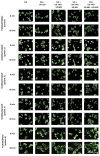
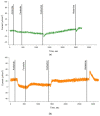
p.Asn1303Lys (N1303K) is a common missense variant of the CFTR gene, causing cystic fibrosis (CF). In this study, we initially evaluated the influence of CFTR modulators on the restoration of N1303K-CFTR function using intestinal organoids derived from four CF patients expressing the N1303K variant. The forskolin-induced swelling assay in organoids offered valuable insights about the beneficial effects of VX-770 + VX-661 + VX-445 (Elexacaftor + Tezacaftor + Ivacaftor, ETI) on N1303K-CFTR function restoration and about discouraging the prescription of VX-770 + VX-809 (Ivacaftor + Lumacaftor) or VX-770 + VX-661 (Ivacaftor + Tezacaftor) therapy for N1303K/class I patients. Then, a comprehensive assessment was conducted on an example of one patient with the N1303K/class I genotype to examine the ETI effect on the restoration of N1303K-CFTR function using in vitro the patient's intestinal organoids, ex vivo the intestinal current measurements (ICM) method and assessment of the clinical status before and after targeted therapy. All obtained results are consistent with each other and have proven the effectiveness of ETI for the N1303K variant. ETI produced a significant positive effect on forskolin-induced swelling in N1303K/class I organoids indicating functional improvement of the CFTR protein; ICM demonstrated that ETI therapy restored CFTR function in the intestinal epithelium after three months of treatment, and the patient improved his clinical status and lung function, increased his body mass index (BMI) and reduced the lung pathogenic flora diversity, surprisingly without improving the sweat test results.
Keywords: BMI; FEV1; N1303K; clinical status; cystic fibrosis; intestinal current measurements; intestinal organoids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
-
N1303K (p.Asn1303Lys) variant: Expanding frontiers in the treatment of cystic fibrosis.Respir Med. 2025 Sep;246:108238. doi: 10.1016/j.rmed.2025.108238. Epub 2025 Jul 9. Respir Med. 2025. PMID: 40645349
-
Personalized CFTR Modulator Therapy for G85E and N1303K Homozygous Patients with Cystic Fibrosis.Int J Mol Sci. 2023 Aug 2;24(15):12365. doi: 10.3390/ijms241512365. Int J Mol Sci. 2023. PMID: 37569738 Free PMC article.
-
VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles.N Engl J Med. 2018 Oct 25;379(17):1612-1620. doi: 10.1056/NEJMoa1807120. Epub 2018 Oct 18. N Engl J Med. 2018. PMID: 30334692 Free PMC article. Clinical Trial.
-
Organic Synthesis and Current Understanding of the Mechanisms of CFTR Modulator Drugs Ivacaftor, Tezacaftor, and Elexacaftor.Molecules. 2024 Feb 10;29(4):821. doi: 10.3390/molecules29040821. Molecules. 2024. PMID: 38398574 Free PMC article. Review.
Cited by
-
Progress of personalized medicine of cystic fibrosis in the times of efficient CFTR modulators.Mol Cell Pediatr. 2025 May 5;12(1):6. doi: 10.1186/s40348-025-00194-0. Mol Cell Pediatr. 2025. PMID: 40320452 Free PMC article. Review.
-
Organoid-on-a-chip (OrgOC): Advancing cystic fibrosis research.Mater Today Bio. 2025 Jul 28;34:102148. doi: 10.1016/j.mtbio.2025.102148. eCollection 2025 Oct. Mater Today Bio. 2025. PMID: 40791795 Free PMC article. Review.
-
Special Issue "Research Advances on Cystic Fibrosis and CFTR Protein".Int J Mol Sci. 2025 Jul 13;26(14):6708. doi: 10.3390/ijms26146708. Int J Mol Sci. 2025. PMID: 40724963 Free PMC article.
References
-
- Cystic Fibrosis Foundations. [(accessed on 21 August 2023)]. Available online: https://www.cff.org/managing-cf/cftr-modulator-therapies.
-
- Mergiotti M., Murabito A., Prono G., Ghigo A. CFTR Modulator Therapy for Rare CFTR Mutants. J. Respir. 2022;2:59–76. doi: 10.3390/jor2020005. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

