Advances in HIV Gene Therapy
- PMID: 38474018
- PMCID: PMC10931721
- DOI: 10.3390/ijms25052771
Advances in HIV Gene Therapy
Abstract
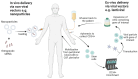
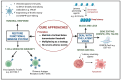
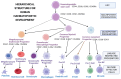
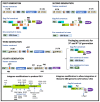
Early gene therapy studies held great promise for the cure of heritable diseases, but the occurrence of various genotoxic events led to a pause in clinical trials and a more guarded approach to progress. Recent advances in genetic engineering technologies have reignited interest, leading to the approval of the first gene therapy product targeting genetic mutations in 2017. Gene therapy (GT) can be delivered either in vivo or ex vivo. An ex vivo approach to gene therapy is advantageous, as it allows for the characterization of the gene-modified cells and the selection of desired properties before patient administration. Autologous cells can also be used during this process which eliminates the possibility of immune rejection. This review highlights the various stages of ex vivo gene therapy, current research developments that have increased the efficiency and safety of this process, and a comprehensive summary of Human Immunodeficiency Virus (HIV) gene therapy studies, the majority of which have employed the ex vivo approach.
Keywords: HIV; ex vivo; gene therapy; stem cells; vector.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ex vivo gene therapy for HIV-1 treatment.Hum Mol Genet. 2011 Apr 15;20(R1):R100-7. doi: 10.1093/hmg/ddr160. Epub 2011 Apr 19. Hum Mol Genet. 2011. PMID: 21505069 Free PMC article. Review.
-
Delivery of gene therapy to resting immune cells for an HIV cure.Curr Opin HIV AIDS. 2019 Mar;14(2):129-136. doi: 10.1097/COH.0000000000000531. Curr Opin HIV AIDS. 2019. PMID: 30608248 Review.
-
Development of HIV vectors for anti-HIV gene therapy.Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11395-9. doi: 10.1073/pnas.93.21.11395. Proc Natl Acad Sci U S A. 1996. PMID: 8876146 Free PMC article. Review.
-
Gene Therapy Approaches to Human Immunodeficiency Virus and Other Infectious Diseases.Hematol Oncol Clin North Am. 2017 Oct;31(5):883-895. doi: 10.1016/j.hoc.2017.06.008. Hematol Oncol Clin North Am. 2017. PMID: 28895854 Review.
-
Safety and efficacy of a tCD25 preselective combination anti-HIV lentiviral vector in human hematopoietic stem and progenitor cells.Stem Cells. 2015 Mar;33(3):870-9. doi: 10.1002/stem.1919. Stem Cells. 2015. PMID: 25524029
Cited by
-
Exploring potential associations between the human microbiota and reservoir of latent HIV.Retrovirology. 2024 Nov 29;21(1):21. doi: 10.1186/s12977-024-00655-w. Retrovirology. 2024. PMID: 39614246 Free PMC article. Review.
-
CRISPR technology in human diseases.MedComm (2020). 2024 Jul 29;5(8):e672. doi: 10.1002/mco2.672. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39081515 Free PMC article. Review.
-
Lymphoblastoid and Jurkat cell lines are useful surrogate in developing a CRISPR-Cas9 method to correct leukocyte adhesion deficiency genomic defect.Front Bioeng Biotechnol. 2025 Mar 21;13:1548227. doi: 10.3389/fbioe.2025.1548227. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40190710 Free PMC article.
-
Deep Thought on the HIV Cured Cases: Where Have We Been and What Lies Ahead?Biomolecules. 2025 Mar 5;15(3):378. doi: 10.3390/biom15030378. Biomolecules. 2025. PMID: 40149913 Free PMC article. Review.
-
Advanced Therapies for Human Immunodeficiency Virus.Med Sci (Basel). 2024 Jul 18;12(3):33. doi: 10.3390/medsci12030033. Med Sci (Basel). 2024. PMID: 39051379 Free PMC article. Review.
References
-
- National Human Genome Research Institute The Human Genome Project Results. The Human Genome Project 2003. [(accessed on 5 July 2022)]; Available online: https://www.genome.gov/human-genome-project/results.
-
- Berdeja J.G., Madduri D., Usmani S.Z., Jakubowiak A., Agha M., Cohen A.D., Stewart A.K., Hari P., Htut M., Lesokhin A., et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet. 2021;398:314–324. doi: 10.1016/S0140-6736(21)00933-8. - DOI - PubMed
-
- Abramson J.S., Palomba M.L., Gordon L.I., Lunning M.A., Wang M., Arnason J., Mehta A., Purev E., Maloney D.G., Andreadis C., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

