Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions
- PMID: 38474022
- PMCID: PMC10932125
- DOI: 10.3390/ijms25052774
Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions
Abstract
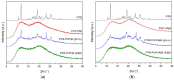
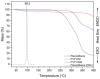
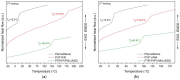
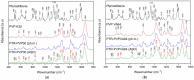
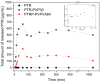
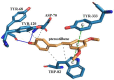
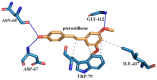
In this study, amorphous solid dispersions (ASDs) of pterostilbene (PTR) with polyvinylpyrrolidone polymers (PVP K30 and VA64) were prepared through milling, affirming the amorphous dispersion of PTR via X-ray powder diffraction (XRPD) and differential scanning calorimetry (DSC). Subsequent analysis of DSC thermograms, augmented using mathematical equations such as the Gordon-Taylor and Couchman-Karasz equations, facilitated the determination of predicted values for glass transition (Tg), PTR's miscibility with PVP, and the strength of PTR's interaction with the polymers. Fourier-transform infrared (FTIR) analysis validated interactions maintaining PTR's amorphous state and identified involved functional groups, namely, the 4'-OH and/or -CH groups of PTR and the C=O group of PVP. The study culminated in evaluating the impact of amorphization on water solubility, the release profile in pH 6.8, and in vitro permeability (PAMPA-GIT and BBB methods). In addition, it was determined how improving water solubility affects the increase in antioxidant (ABTS, DPPH, CUPRAC, and FRAP assays) and neuroprotective (inhibition of cholinesterases: AChE and BChE) properties. The apparent solubility of the pure PTR was ~4.0 µg·mL-1 and showed no activity in the considered assays. For obtained ASDs (PTR-PVP30/PTR-PVPVA64, respectively) improvements in apparent solubility (410.8 and 383.2 µg·mL-1), release profile, permeability, antioxidant properties (ABTS: IC50 = 52.37/52.99 μg·mL-1, DPPH: IC50 = 163.43/173.96 μg·mL-1, CUPRAC: IC0.5 = 122.27/129.59 μg·mL-1, FRAP: IC0.5 = 95.69/98.57 μg·mL-1), and neuroprotective effects (AChE: 39.1%/36.2%, BChE: 76.9%/73.2%) were confirmed.
Keywords: Couchman–Karasz equation; Gordon–Taylor equation; amorphous solid dispersion; antioxidation; glass transition; miscibility; molecular modeling; neuroprotection; polyvinylpyrrolidone; pterostilbene.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
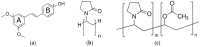
Similar articles
-
Mechanochemical Approach to Obtaining a Multicomponent Fisetin Delivery System Improving Its Solubility and Biological Activity.Int J Mol Sci. 2024 Mar 25;25(7):3648. doi: 10.3390/ijms25073648. Int J Mol Sci. 2024. PMID: 38612460 Free PMC article.
-
Amorphous Pterostilbene Delivery Systems Preparation-Innovative Approach to Preparation Optimization.Pharmaceutics. 2023 Apr 13;15(4):1231. doi: 10.3390/pharmaceutics15041231. Pharmaceutics. 2023. PMID: 37111715 Free PMC article.
-
Myricetin Amorphous Solid Dispersions-Antineurodegenerative Potential.Molecules. 2024 Mar 14;29(6):1287. doi: 10.3390/molecules29061287. Molecules. 2024. PMID: 38542923 Free PMC article.
-
A comparison of spray drying and milling in the production of amorphous dispersions of sulfathiazole/polyvinylpyrrolidone and sulfadimidine/polyvinylpyrrolidone.Mol Pharm. 2011 Apr 4;8(2):532-42. doi: 10.1021/mp1003674. Epub 2011 Mar 3. Mol Pharm. 2011. PMID: 21323367
-
Phase behavior, intermolecular interaction, and solid state characterization of amorphous solid dispersion of Febuxostat.Pharm Dev Technol. 2017 Feb;22(1):45-57. doi: 10.3109/10837450.2016.1138130. Epub 2016 Feb 5. Pharm Dev Technol. 2017. PMID: 26853838
Cited by
-
Enhancing the Solubility and Dissolution of Apigenin: Solid Dispersions Approach.Int J Mol Sci. 2025 Jan 10;26(2):566. doi: 10.3390/ijms26020566. Int J Mol Sci. 2025. PMID: 39859284 Free PMC article.
-
Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence.Metabolites. 2025 Feb 13;15(2):124. doi: 10.3390/metabo15020124. Metabolites. 2025. PMID: 39997749 Free PMC article. Review.
-
The Interplay Between Autophagy and Apoptosis in the Mechanisms of Action of Stilbenes in Cancer Cells.Antioxidants (Basel). 2025 Mar 13;14(3):339. doi: 10.3390/antiox14030339. Antioxidants (Basel). 2025. PMID: 40227400 Free PMC article. Review.
-
Mechanochemical Approach to Obtaining a Multicomponent Fisetin Delivery System Improving Its Solubility and Biological Activity.Int J Mol Sci. 2024 Mar 25;25(7):3648. doi: 10.3390/ijms25073648. Int J Mol Sci. 2024. PMID: 38612460 Free PMC article.
-
Curcumin Solubility and Bioactivity Enhancement Through Amorphization with Tryptophan via Supercritical Fluid Technology.Int J Mol Sci. 2025 Jan 20;26(2):855. doi: 10.3390/ijms26020855. Int J Mol Sci. 2025. PMID: 39859569 Free PMC article.
References
-
- Huang W.-C., Liu J.-C., Hsia C.-W., Fong T.-H., Hsia C.-H., Tran O.-T., Velusamy M., Yang C.-H., Sheu J.-R. Pterostilbene, a Dimethylether Analogue of Resveratrol, Possesses High Potency in the Prevention of Platelet Activation in Humans and the Reduction of Vascular Thrombosis in Mice. J. Agric. Food Chem. 2021;69:4697–4707. doi: 10.1021/acs.jafc.1c00367. - DOI - PubMed
-
- Gómez-Zorita S., González-Arceo M., Trepiana J., Aguirre L., Crujeiras A.B., Irles E., Segues N., Bujanda L., Portillo M.P. Comparative Effects of Pterostilbene and Its Parent Compound Resveratrol on Oxidative Stress and Inflammation in Steatohepatitis Induced by High-Fat High-Fructose Feeding. Antioxidants. 2020;9:1042. doi: 10.3390/antiox9111042. - DOI - PMC - PubMed
-
- Chiou Y.-S., Tsai M.-L., Nagabhushanam K., Wang Y.-J., Wu C.-H., Ho C.-T., Pan M.-H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

