Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions
- PMID: 38474022
- PMCID: PMC10932125
- DOI: 10.3390/ijms25052774
Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions
Abstract
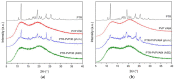
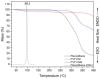
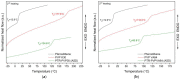
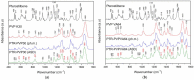
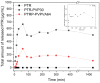
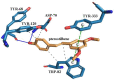
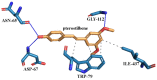
In this study, amorphous solid dispersions (ASDs) of pterostilbene (PTR) with polyvinylpyrrolidone polymers (PVP K30 and VA64) were prepared through milling, affirming the amorphous dispersion of PTR via X-ray powder diffraction (XRPD) and differential scanning calorimetry (DSC). Subsequent analysis of DSC thermograms, augmented using mathematical equations such as the Gordon-Taylor and Couchman-Karasz equations, facilitated the determination of predicted values for glass transition (Tg), PTR's miscibility with PVP, and the strength of PTR's interaction with the polymers. Fourier-transform infrared (FTIR) analysis validated interactions maintaining PTR's amorphous state and identified involved functional groups, namely, the 4'-OH and/or -CH groups of PTR and the C=O group of PVP. The study culminated in evaluating the impact of amorphization on water solubility, the release profile in pH 6.8, and in vitro permeability (PAMPA-GIT and BBB methods). In addition, it was determined how improving water solubility affects the increase in antioxidant (ABTS, DPPH, CUPRAC, and FRAP assays) and neuroprotective (inhibition of cholinesterases: AChE and BChE) properties. The apparent solubility of the pure PTR was ~4.0 µg·mL-1 and showed no activity in the considered assays. For obtained ASDs (PTR-PVP30/PTR-PVPVA64, respectively) improvements in apparent solubility (410.8 and 383.2 µg·mL-1), release profile, permeability, antioxidant properties (ABTS: IC50 = 52.37/52.99 μg·mL-1, DPPH: IC50 = 163.43/173.96 μg·mL-1, CUPRAC: IC0.5 = 122.27/129.59 μg·mL-1, FRAP: IC0.5 = 95.69/98.57 μg·mL-1), and neuroprotective effects (AChE: 39.1%/36.2%, BChE: 76.9%/73.2%) were confirmed.
Keywords: Couchman–Karasz equation; Gordon–Taylor equation; amorphous solid dispersion; antioxidation; glass transition; miscibility; molecular modeling; neuroprotection; polyvinylpyrrolidone; pterostilbene.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
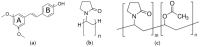
References
-
- Huang W.-C., Liu J.-C., Hsia C.-W., Fong T.-H., Hsia C.-H., Tran O.-T., Velusamy M., Yang C.-H., Sheu J.-R. Pterostilbene, a Dimethylether Analogue of Resveratrol, Possesses High Potency in the Prevention of Platelet Activation in Humans and the Reduction of Vascular Thrombosis in Mice. J. Agric. Food Chem. 2021;69:4697–4707. doi: 10.1021/acs.jafc.1c00367. - DOI - PubMed
-
- Gómez-Zorita S., González-Arceo M., Trepiana J., Aguirre L., Crujeiras A.B., Irles E., Segues N., Bujanda L., Portillo M.P. Comparative Effects of Pterostilbene and Its Parent Compound Resveratrol on Oxidative Stress and Inflammation in Steatohepatitis Induced by High-Fat High-Fructose Feeding. Antioxidants. 2020;9:1042. doi: 10.3390/antiox9111042. - DOI - PMC - PubMed
-
- Chiou Y.-S., Tsai M.-L., Nagabhushanam K., Wang Y.-J., Wu C.-H., Ho C.-T., Pan M.-H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

