Plasma-Activated Polydimethylsiloxane Microstructured Pattern with Collagen for Improved Myoblast Cell Guidance
- PMID: 38474025
- PMCID: PMC10932060
- DOI: 10.3390/ijms25052779
Plasma-Activated Polydimethylsiloxane Microstructured Pattern with Collagen for Improved Myoblast Cell Guidance
Abstract
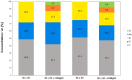
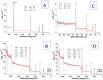
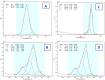
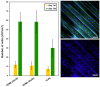
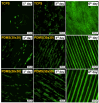
We focused on polydimethylsiloxane (PDMS) as a substrate for replication, micropatterning, and construction of biologically active surfaces. The novelty of this study is based on the combination of the argon plasma exposure of a micropatterned PDMS scaffold, where the plasma served as a strong tool for subsequent grafting of collagen coatings and their application as cell growth scaffolds, where the standard was significantly exceeded. As part of the scaffold design, templates with a patterned microstructure of different dimensions (50 × 50, 50 × 20, and 30 × 30 μm2) were created by photolithography followed by pattern replication on a PDMS polymer substrate. Subsequently, the prepared microstructured PDMS replicas were coated with a type I collagen layer. The sample preparation was followed by the characterization of material surface properties using various analytical techniques, including scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS). To evaluate the biocompatibility of the produced samples, we conducted studies on the interactions between selected polymer replicas and micro- and nanostructures and mammalian cells. Specifically, we utilized mouse myoblasts (C2C12), and our results demonstrate that we achieved excellent cell alignment in conjunction with the development of a cytocompatible surface. Consequently, the outcomes of this research contribute to an enhanced comprehension of surface properties and interactions between structured polymers and mammalian cells. The use of periodic microstructures has the potential to advance the creation of novel materials and scaffolds in tissue engineering. These materials exhibit exceptional biocompatibility and possess the capacity to promote cell adhesion and growth.
Keywords: PDMS; coating; collagen type I; cytocompatibility; microstructure; myoblast cell; nanostructured pattern; replication; soft lithography.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Polydopamine-collagen complex to enhance the biocompatibility of polydimethylsiloxane substrates for sustaining long-term culture of L929 fibroblasts and tendon stem cells.J Biomed Mater Res A. 2018 Feb;106(2):408-418. doi: 10.1002/jbm.a.36254. Epub 2017 Oct 17. J Biomed Mater Res A. 2018. PMID: 28971550
-
Selective cell adhesion on femtosecond laser-microstructured polydimethylsiloxane.Biomed Mater. 2016 Feb 19;11(1):015014. doi: 10.1088/1748-6041/11/1/015014. Biomed Mater. 2016. PMID: 26894472
-
Chemical and physical modifications to poly(dimethylsiloxane) surfaces affect adhesion of Caco-2 cells.J Biomed Mater Res A. 2010 Jun 15;93(4):1260-71. doi: 10.1002/jbm.a.32621. J Biomed Mater Res A. 2010. PMID: 19827104
-
Advancing Organ-on-a-Chip Systems: The Role of Scaffold Materials and Coatings in Engineering Cell Microenvironment.Polymers (Basel). 2025 May 6;17(9):1263. doi: 10.3390/polym17091263. Polymers (Basel). 2025. PMID: 40363048 Free PMC article. Review.
-
Properties and Applications of PDMS for Biomedical Engineering: A Review.J Funct Biomater. 2021 Dec 21;13(1):2. doi: 10.3390/jfb13010002. J Funct Biomater. 2021. PMID: 35076525 Free PMC article. Review.
Cited by
-
Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation.Polymers (Basel). 2025 Mar 20;17(6):822. doi: 10.3390/polym17060822. Polymers (Basel). 2025. PMID: 40292716 Free PMC article. Review.
-
Synergistic effects of micropatterned substrates and transforming growth factor-β1 on differentiation of human mesenchymal stem cells into vascular smooth muscle cells through modulation of Krϋppel-like factor 4.In Vitro Cell Dev Biol Anim. 2025 Jun;61(6):644-655. doi: 10.1007/s11626-025-01033-2. Epub 2025 May 23. In Vitro Cell Dev Biol Anim. 2025. PMID: 40408054 Free PMC article.
-
Macrophage Immunomodulation and Suppression of Bacterial Growth by Polydimethylsiloxane Surface-Interrupted Microlines' Topography Targeting Breast Implant Applications.Polymers (Basel). 2024 Oct 29;16(21):3046. doi: 10.3390/polym16213046. Polymers (Basel). 2024. PMID: 39518255 Free PMC article.
-
Influence of Soft and Stiff Matrices on Cytotoxicity in Gingival Fibroblasts: Implications for Soft Tissue Biocompatibility.Cells. 2024 Nov 21;13(23):1932. doi: 10.3390/cells13231932. Cells. 2024. PMID: 39682682 Free PMC article.
References
-
- Rajendran A.K., Kim H.D., Kim J.-W., Bae J.W., Hwang N.S. Nanotechnology in tissue engineering and regenerative medicine. Korean J. Chem. Eng. 2023;40:286–301. doi: 10.1007/s11814-022-1363-1. - DOI
-
- Kirkpatrick C.J., Mittermayer C. Theoretical and practical aspects of testing potential biomaterials in vitro. J. Mater. Sci. Mater. Med. 1990;1:9–13. doi: 10.1007/BF00705347. - DOI
-
- De Jong W.H., Carraway J.W., Geertsma R.E. Chapter 6—In vivo and in vitro testing for the biological safety evaluation of biomaterials and medical devices. In: Boutrand J.P., editor. Biocompatibility and Performance of Medical Devices. 2nd ed. Woodhead Publishing; Sawston, UK: 2020. pp. 123–166.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

