Mass Spectrometry-Based Proteomic Analysis of Potential Host Proteins Interacting with GP5 in PRRSV-Infected PAMs
- PMID: 38474030
- PMCID: PMC10932240
- DOI: 10.3390/ijms25052778
Mass Spectrometry-Based Proteomic Analysis of Potential Host Proteins Interacting with GP5 in PRRSV-Infected PAMs
Abstract
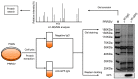
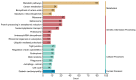
Porcine reproductive and respiratory syndrome virus (PRRSV) is a typical immunosuppressive virus causing a large economic impact on the swine industry. The structural protein GP5 of PRRSV plays a pivotal role in its pathogenicity and immune evasion. Virus-host interactions play a crucial part in viral replication and immune escape. Therefore, understanding the interactions between GP5 and host proteins are significant for porcine reproductive and respiratory syndrome (PRRS) control. However, the interaction network between GP5 and host proteins in primary porcine alveolar macrophages (PAMs) has not been reported. In this study, 709 GP5-interacting host proteins were identified in primary PAMs by immunoprecipitation coupled with liquid chromatography-tandem mass spectrometry (LC-MS/MS). Bioinformatics analysis revealed that these proteins were involved in multiple cellular processes, such as translation, protein transport, and protein stabilization. Subsequently, immunoprecipitation and immunofluorescence assay confirmed that GP5 could interact with antigen processing and presentation pathways related proteins. Finally, we found that GP5 may be a key protein that inhibits the antigen processing and presentation pathway during PRRSV infection. The novel host proteins identified in this study will be the candidates for studying the biological functions of GP5, which will provide new insights into PRRS prevention and vaccine development.
Keywords: GP5; LC-MS/MS; PRRSV; antigen processing and presentation; protein–protein interaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Zhou L.J., Yang Y., Xia Q.Q., Guan Z.X., Zhang J.J., Li B.B., Qiu Y.F., Liu K., Shao D.H., Ma Z.Y., et al. Genetic characterization of porcine reproductive and respiratory syndrome virus from Eastern China during 2017–2022. Front. Microbiol. 2022;13:971817. doi: 10.3389/fmicb.2022.971817. - DOI - PMC - PubMed
-
- Zhang L.J., Feng X., Wang H.D., He S.J., Fan H.J., Liu D.Y. Antibody-dependent enhancement of porcine reproductive and respiratory syndrome virus infection downregulates the levels of interferon-gamma/lambdas in porcine alveolar macrophages in vitro. Front. Vet. Sci. 2023;10:1150430. doi: 10.3389/fvets.2023.1150430. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

