The Role of Transglutaminase 2 in Cancer: An Update
- PMID: 38474044
- PMCID: PMC10931703
- DOI: 10.3390/ijms25052797
The Role of Transglutaminase 2 in Cancer: An Update
Abstract
Transglutaminase type 2 (TG2) is the most ubiquitously expressed and well characterized member of the transglutaminase family. It is a ubiquitous multifunctional enzyme implicated in the regulation of several cellular pathways that support the survival, death, and general homeostasis of eukaryotic cells. Due to its multiple localizations both inside and outside the cell, TG2 participates in the regulation of many crucial intracellular signaling cascades in a tissue- and cell-specific manner, making this enzyme an important player in disease development and progression. Moreover, TG2 is capable of modulating the tumor microenvironment, a process of dynamic tissue remodeling and biomechanical events, resulting in changes which influence tumor initiation, growth, and metastasis. Even if generally related to the Ca2+-dependent post-translational modification of proteins, a number of different biological functions have been ascribed to TG2, like those of a peptide isomerase, protein kinase, guanine nucleotide binder, and cytosolic-nuclear translocator. With respect to cancer, TG2's role is controversial and highly debated; it has been described both as an anti- and pro-apoptotic factor and is linked to all the processes of tumorigenesis. However, numerous pieces of evidence support a tissue-specific role of TG2 so that it can assume both oncogenic and tumor-suppressive roles.
Keywords: breast cancer; colorectal cancer; leukemia; lung cancer; melanoma; microenvironment; ovarian cancer; pancreatic cancer; transglutaminase 2.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
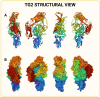
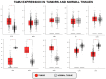
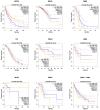
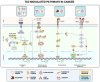


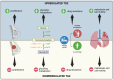

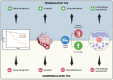
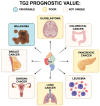
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

