Human Papilloma Virus Positive Oropharyngeal Squamous Cell Carcinoma and the Immune System: Pathogenesis, Immunotherapy and Future Perspectives
- PMID: 38474047
- PMCID: PMC10932043
- DOI: 10.3390/ijms25052798
Human Papilloma Virus Positive Oropharyngeal Squamous Cell Carcinoma and the Immune System: Pathogenesis, Immunotherapy and Future Perspectives
Abstract
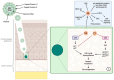
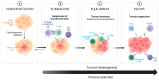
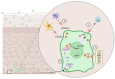
Oropharyngeal squamous cell carcinoma (OPSCC), a subset of head and neck squamous cell carcinoma (HNSCC), involves the palatine tonsils, soft palate, base of tongue, and uvula, with the ability to spread to adjacent subsites. Personalized treatment strategies for Human Papillomavirus-associated squamous cell carcinoma of the oropharynx (HPV+OPSCC) are yet to be established. In this article, we summarise our current understanding of the pathogenesis of HPV+OPSCC, the intrinsic role of the immune system, current ICI clinical trials, and the potential role of small molecule immunotherapy in HPV+OPSCC.
Keywords: cancer of the oropharynx; head & neck cancer; immune system; immunotherapy.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Seiwert T.Y., Zuo Z., Keck M.K., Khattri A., Pedamallu C.S., Stricker T., Brown C., Pugh T.J., Stojanov P., Cho J., et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 2015;21:632–641. doi: 10.1158/1078-0432.CCR-13-3310. - DOI - PMC - PubMed
-
- Chung C.H., Guthrie V.B., Masica D.L., Tokheim C., Kang H., Richmon J., Agrawal N., Fakhry C., Quon H., Subramaniam R.M., et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann. Oncol. 2015;26:1216–1223. doi: 10.1093/annonc/mdv109. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

