Interleukin-6: An Under-Appreciated Inducer of Thermogenic Adipocyte Differentiation
- PMID: 38474057
- PMCID: PMC10932467
- DOI: 10.3390/ijms25052810
Interleukin-6: An Under-Appreciated Inducer of Thermogenic Adipocyte Differentiation
Abstract
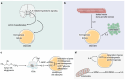
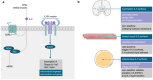
Adipose tissue inflammation is a key factor leading to obesity-associated immune disorders, such as insulin resistance, beta cell loss in the pancreatic islets, meta-inflammation, and autoimmunity. Inhibiting adipose tissue inflammation is considered a straightforward approach to abrogate these diseases. However, recent findings show that certain pro-inflammatory cytokines are essential for the proper differentiation and functioning of adipocytes. Lipolysis is stimulated, and the thermogenic competence of adipocytes is unlocked by interleukin-6 (IL-6), a cytokine that was initially recognized as a key trigger of adipose tissue inflammation. Coherently, signal transducer and activator of transcription 3 (STAT3), which is a signal transducer for IL-6, is necessary for thermogenic adipocyte development. Given the impact of thermogenic adipocytes in increasing energy expenditure and reducing body adiposity, functions of IL-6 in the adipose tissue have gained attention recently. In this review, we show that IL-6 signaling may protect from excess fat accumulation by stimulating thermogenesis in adipocytes.
Keywords: adipocyte; inflammation; obesity; thermogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Effects of aerobic exercise on the inflammatory cytokine profile and expression of lipolytic and thermogenic genes in β1-AR-/- mice adipose tissue.Life Sci. 2019 Mar 15;221:224-232. doi: 10.1016/j.lfs.2019.02.031. Epub 2019 Feb 13. Life Sci. 2019. PMID: 30771314
-
Inhibition of STAT3 enhances UCP1 expression and mitochondrial function in brown adipocytes.Eur J Pharmacol. 2022 Jul 5;926:175040. doi: 10.1016/j.ejphar.2022.175040. Epub 2022 May 19. Eur J Pharmacol. 2022. PMID: 35598846
-
Inflammatory Signaling and Brown Fat Activity.Front Endocrinol (Lausanne). 2020 Mar 24;11:156. doi: 10.3389/fendo.2020.00156. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32265845 Free PMC article. Review.
-
Augmented CCL5/CCR5 signaling in brown adipose tissue inhibits adaptive thermogenesis and worsens insulin resistance in obesity.Clin Sci (Lond). 2022 Jan 14;136(1):121-137. doi: 10.1042/CS20210959. Clin Sci (Lond). 2022. PMID: 34821367
-
Regulation of adipocyte thermogenesis: mechanisms controlling obesity.FEBS J. 2020 Aug;287(16):3370-3385. doi: 10.1111/febs.15331. Epub 2020 Apr 27. FEBS J. 2020. PMID: 32301220 Review.
Cited by
-
Adipose Tissue Macrophages of the Human Fetus.Cells. 2024 Oct 28;13(21):1787. doi: 10.3390/cells13211787. Cells. 2024. PMID: 39513894 Free PMC article.
-
HIV infection drives proinflammatory adipocyte differentiation in an in vitro model and reveals a new inflammatory pathway.Front Cell Infect Microbiol. 2025 Jul 17;15:1627963. doi: 10.3389/fcimb.2025.1627963. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40746960 Free PMC article.
References
-
- Roglic G. WHO Global report on diabetes: A summary. Int. J. Noncommun. Dis. 2016;1:3–8. doi: 10.4103/2468-8827.184853. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- NKFI-OTKA 142939/Hungarian Research Fund
- na/Bolyai Research Scholarship of the Hungarian Academy of Sciences
- na/Dean's Research Fund (Faculty of Medicine, University of Debrecen)
- na/"Momentum" proof-of-concept fund (Faculty of Medicine, University of Debrecen)
- UNKP-23-5/New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund.
LinkOut - more resources
Full Text Sources
Miscellaneous

