Anabolic Steroids Activate the NF-κB Pathway in Porcine Ovarian Putative Stem Cells Independently of the ZIP-9 Receptor
- PMID: 38474077
- PMCID: PMC10932267
- DOI: 10.3390/ijms25052833
Anabolic Steroids Activate the NF-κB Pathway in Porcine Ovarian Putative Stem Cells Independently of the ZIP-9 Receptor
Abstract
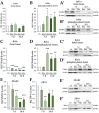
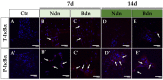
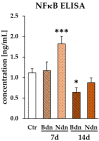
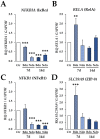
Boldenone (Bdn) and nandrolone (Ndn) are anabolic androgenic steroids (AASs) that, as our previous studies have shown, may increase the risk of neoplastic transformation of porcine ovarian putative stem cells (poPSCs). The NF-κB pathway may be important in the processes of carcinogenesis and tumour progression. Therefore, in this work, we decided to test the hypothesis of whether Bdn and Ndn can activate the NF-κB pathway by acting through the membrane androgen receptor ZIP-9. For this purpose, the expression profiles of both genes involved in the NF-κB pathway and the gene coding for the ZIP-9 receptor were checked. The expression and localization of proteins of this pathway in poPSCs were also examined. Additionally, the expression of the ZIP-9 receptor and the concentration of the NF-κB1 and 2 protein complex were determined. Activation of the NF-κB pathway was primarily confirmed by an increase in the relative abundances of phosphorylated forms of RelA protein and IκBα inhibitor. Reduced quantitative profiles pinpointed not only for genes representing this pathway but also for unphosphorylated proteins, and, simultaneously, decreased concentration of the NF-κB1 and 2 complex may indicate post-activation silencing by negative feedback. However, the remarkably and sustainably diminished expression levels noticed for the SLC39A9 gene and ZIP-9 protein suggest that this receptor does not play an important role in the regulation of the NF-κB pathway.
Keywords: NF-κB signalling pathway; ZIP-9; boldenone; nandrolone; ovary; pig; putative stem cells.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

