Biomolecular Actions by Intestinal Endotoxemia in Metabolic Syndrome
- PMID: 38474087
- PMCID: PMC10931779
- DOI: 10.3390/ijms25052841
Biomolecular Actions by Intestinal Endotoxemia in Metabolic Syndrome
Abstract
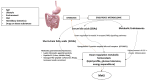
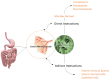
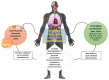
Metabolic syndrome (MetS) is a combination of metabolic disorders that concurrently act as factors promoting systemic pathologies such as atherosclerosis or diabetes mellitus. It is now believed to encompass six main interacting conditions: visceral fat, imbalance of lipids (dyslipidemia), hypertension, insulin resistance (with or without impairing both glucose tolerance and fasting blood sugar), and inflammation. In the last 10 years, there has been a progressive interest through scientific research investigations conducted in the field of metabolomics, confirming a trend to evaluate the role of the metabolome, particularly the intestinal one. The intestinal microbiota (IM) is crucial due to the diversity of microorganisms and their abundance. Consequently, IM dysbiosis and its derivate toxic metabolites have been correlated with MetS. By intervening in these two factors (dysbiosis and consequently the metabolome), we can potentially prevent or slow down the clinical effects of the MetS process. This, in turn, may mitigate dysregulations of intestinal microbiota axes, such as the lung axis, thereby potentially alleviating the negative impact on respiratory pathology, such as the chronic obstructive pulmonary disease. However, the biomolecular mechanisms through which the IM influences the host's metabolism via a dysbiosis metabolome in both normal and pathological conditions are still unclear. In this study, we seek to provide a description of the knowledge to date of the IM and its metabolome and the factors that influence it. Furthermore, we analyze the interactions between the functions of the IM and the pathophysiology of major metabolic diseases via local and systemic metabolome's relate endotoxemia.
Keywords: biochemistry; chronic obstructive pulmonary disease (COPD); human microbiota; immunity; metabolic syndrome (MetS); metabolome; microbiota’s crosstalk axis; molecular biology; prebiotics; probiotics; respiratory rehabilitation.
Conflict of interest statement
The authors report no competing interests to declare.
Figures








Similar articles
-
Intestinal Microbiota Dysbiosis Role and Bacterial Translocation as a Factor for Septic Risk.Int J Mol Sci. 2025 Feb 26;26(5):2028. doi: 10.3390/ijms26052028. Int J Mol Sci. 2025. PMID: 40076650 Free PMC article. Review.
-
[Physiological patterns of intestinal microbiota. The role of dysbacteriosis in obesity, insulin resistance, diabetes and metabolic syndrome].Orv Hetil. 2016 Jan 3;157(1):13-22. doi: 10.1556/650.2015.30296. Orv Hetil. 2016. PMID: 26708682 Review. Hungarian.
-
Dietary Strategies for Management of Metabolic Syndrome: Role of Gut Microbiota Metabolites.Nutrients. 2021 Apr 21;13(5):1389. doi: 10.3390/nu13051389. Nutrients. 2021. PMID: 33919016 Free PMC article. Review.
-
Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects With Metabolic Syndrome.J Am Heart Assoc. 2019 Sep 3;8(17):e012401. doi: 10.1161/JAHA.119.012401. Epub 2019 Aug 27. J Am Heart Assoc. 2019. PMID: 31451009 Free PMC article. Clinical Trial.
-
Metabolic endotoxemia and cardiovascular disease: A systematic review about potential roles of prebiotics and probiotics.Clin Exp Pharmacol Physiol. 2020 Jun;47(6):927-939. doi: 10.1111/1440-1681.13250. Epub 2020 Jan 24. Clin Exp Pharmacol Physiol. 2020. PMID: 31894861
Cited by
-
The Role of Diet, Additives, and Antibiotics in Metabolic Endotoxemia and Chronic Diseases.Metabolites. 2024 Dec 13;14(12):704. doi: 10.3390/metabo14120704. Metabolites. 2024. PMID: 39728485 Free PMC article. Review.
-
Intestinal Microbiota Dysbiosis Role and Bacterial Translocation as a Factor for Septic Risk.Int J Mol Sci. 2025 Feb 26;26(5):2028. doi: 10.3390/ijms26052028. Int J Mol Sci. 2025. PMID: 40076650 Free PMC article. Review.
-
Association between lipid accumulation product and chronic obstructive pulmonary disease: a cross-sectional study based on U.S. adults.Front Nutr. 2025 Jan 10;11:1517108. doi: 10.3389/fnut.2024.1517108. eCollection 2024. Front Nutr. 2025. PMID: 39867561 Free PMC article.
-
The Role of Grifola frondosa Polysaccharide in Preventing Skeletal Muscle Atrophy in Type 2 Diabetes Mellitus.Life (Basel). 2024 Jun 21;14(7):784. doi: 10.3390/life14070784. Life (Basel). 2024. PMID: 39063539 Free PMC article.
-
Endothelial Dysfunction and Liver Cirrhosis: Unraveling of a Complex Relationship.Int J Mol Sci. 2024 Nov 29;25(23):12859. doi: 10.3390/ijms252312859. Int J Mol Sci. 2024. PMID: 39684569 Free PMC article. Review.
References
-
- Noubiap J.J., Nansseu J.R., Lontchi-Yimagou E., Nkeck J.R., Nyaga U.F., Ngouo A.T., Tounouga D.N., Tianyi F.L., Foka A.J., Ndoadoumgue A.L., et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022;188:109924. doi: 10.1016/j.diabres.2022.109924. - DOI - PubMed
-
- Palmnäs-Bédard M.S., Costabile G., Vetrani C., Åberg S., Hjalmarsson Y., Dicksved J., Riccardi G., Landberg R. The human gut microbiota and glucose metabolism: A scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022;116:862–874. doi: 10.1093/ajcn/nqac217. - DOI - PMC - PubMed
-
- Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., De Vadder F., Arora T., Hallen A., Martens E., Björck I., Bäckhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

