Inhibition of PRMT5/MEP50 Arginine Methyltransferase Activity Causes Cancer Vulnerability in NDRG2low Adult T-Cell Leukemia/Lymphoma
- PMID: 38474089
- PMCID: PMC10932150
- DOI: 10.3390/ijms25052842
Inhibition of PRMT5/MEP50 Arginine Methyltransferase Activity Causes Cancer Vulnerability in NDRG2low Adult T-Cell Leukemia/Lymphoma
Abstract
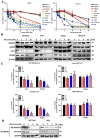
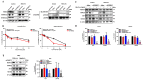
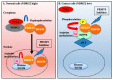
N-myc downstream-regulated gene 2 (NDRG2), which is a tumour suppressor, is frequently lost in many types of tumours, including adult T-cell leukaemia/lymphoma (ATL). The downregulation of NDRG2 expression is involved in tumour progression through the aberrant phosphorylation of several important signalling molecules. We observed that the downregulation of NDRG2 induced the translocation of protein arginine methyltransferase 5 (PRMT5) from the nucleus to the cytoplasm via the increased phosphorylation of PRMT5 at Serine 335. In NDRG2low ATL, cytoplasmic PRMT5 enhanced HSP90A chaperone activity via arginine methylation, leading to tumour progression and the maintenance of oncogenic client proteins. Therefore, we examined whether the inhibition of PRMT5 activity is a drug target in NDRG2low tumours. The knockdown of PRMT5 and binding partner methylsome protein 50 (MEP50) expression significantly demonstrated the suppression of cell proliferation via the degradation of AKT and NEMO in NDRG2low ATL cells, whereas NDRG2-expressing cells did not impair the stability of client proteins. We suggest that the relationship between PRMT5/MEP50 and the downregulation of NDRG2 may exhibit a novel vulnerability and a therapeutic target. Treatment with the PRMT5-specific inhibitors CMP5 and HLCL61 was more sensitive in NDRG2low cancer cells than in NDRG2-expressing cells via the inhibition of HSP90 arginine methylation, along with the degradation of client proteins. Thus, interference with PRMT5 activity has become a feasible and effective strategy for promoting cancer vulnerability in NDRG2low ATL.
Keywords: ATL; MEP50; NDRG2; PRMT5; cancer vulnerability.
Conflict of interest statement
T.M. has financial interests in Denka Co., Ltd. T.I., A.S., S.N., H.I., K.S. and K.M. declare no competing interests in this article.
Figures





References
-
- Einsiedel L., Pham H., Talukder M.R.R., Liddle J., Taylor K., Wilson K., Jersmann H., Gessain A., Woodman R., Kaldor J. Pulmonary Disease Is Associated With Human T-Cell Leukemia Virus Type 1c Infection: A Cross-sectional Survey in Remote Aboriginal Communities. Clin. Infect. Dis. 2020;73:e1498–e1506. doi: 10.1093/cid/ciaa1401. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

