Role of PKN1 in Retinal Cell Type Formation
- PMID: 38474095
- PMCID: PMC10931565
- DOI: 10.3390/ijms25052848
Role of PKN1 in Retinal Cell Type Formation
Abstract
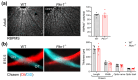
We recently identified PKN1 as a developmentally active gatekeeper of the transcription factor neuronal differentiation-2 (NeuroD2) in several brain areas. Since NeuroD2 plays an important role in amacrine cell (AC) and retinal ganglion cell (RGC) type formation, we aimed to study the expression of NeuroD2 in the postnatal retina of WT and Pkn1-/- animals, with a particular focus on these two cell types. We show that PKN1 is broadly expressed in the retina and that the gross retinal structure is not different between both genotypes. Postnatal retinal NeuroD2 levels were elevated upon Pkn1 knockout, with Pkn1-/- retinae showing more NeuroD2+ cells in the lower portion of the inner nuclear layer. Accordingly, immunohistochemical analysis revealed an increased amount of AC in postnatal and adult Pkn1-/- retinae. There were no differences in horizontal cell, bipolar cell, glial cell and RGC numbers, nor defective axon guidance to the optic chiasm or tract upon Pkn1 knockout. Interestingly, we did, however, see a specific reduction in SMI-32+ α-RGC in Pkn1-/- retinae. These results suggest that PKN1 is important for retinal cell type formation and validate PKN1 for future studies focusing on AC and α-RGC specification and development.
Keywords: NeuroD2; amacrine cells; protein kinase N1; retinal development; retinal ganglion cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
PKN1 Is a Novel Regulator of Hippocampal GluA1 Levels.Front Synaptic Neurosci. 2021 Feb 5;13:640495. doi: 10.3389/fnsyn.2021.640495. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 33613259 Free PMC article.
-
Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina.J Comp Neurol. 2003 Jun 23;461(2):187-204. doi: 10.1002/cne.10674. J Comp Neurol. 2003. PMID: 12724837
-
Expression of LIM-homeodomain transcription factors in the developing and mature mouse retina.Gene Expr Patterns. 2014 Jan;14(1):1-8. doi: 10.1016/j.gep.2013.12.001. Epub 2013 Dec 10. Gene Expr Patterns. 2014. PMID: 24333658 Free PMC article.
-
NeuroD factors regulate cell fate and neurite stratification in the developing retina.J Neurosci. 2011 May 18;31(20):7365-79. doi: 10.1523/JNEUROSCI.2555-10.2011. J Neurosci. 2011. PMID: 21593321 Free PMC article.
-
Cell-type specification in the retina: Recent discoveries from transcriptomic approaches.Curr Opin Neurobiol. 2023 Aug;81:102752. doi: 10.1016/j.conb.2023.102752. Epub 2023 Jul 25. Curr Opin Neurobiol. 2023. PMID: 37499619 Review.
References
-
- Goetz J., Jessen Z.F., Jacobi A., Mani A., Cooler S., Greer D., Kadri S., Segal J., Shekhar K., Sanes J.R., et al. Unified classification of mouse retinal ganglion cells using function, morphology, and gene expression. Cell Rep. 2022;40:111040. doi: 10.1016/j.celrep.2022.111040. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

