The Relevance, Predictability, and Utility of Annexin A5 for Human Physiopathology
- PMID: 38474114
- PMCID: PMC10932194
- DOI: 10.3390/ijms25052865
The Relevance, Predictability, and Utility of Annexin A5 for Human Physiopathology
Abstract
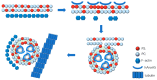
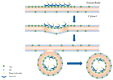
As an important functional protein molecule in the human body, human annexin A5 (hAnxA5) is widely found in human cells and body fluids. hAnxA5, the smallest type of annexin, performs a variety of biological functions by reversibly and specifically binding phosphatidylserine (PS) in a calcium-dependent manner and plays an important role in many human physiological and pathological processes. The free state hAnxA5 exists in the form of monomers and usually forms a polymer in a specific self-assembly manner when exerting biological activity. This review systematically discusses the current knowledge and understanding of hAnxA5 from three perspectives: physiopathological relevance, diagnostic value, and therapeutic utility. hAnxA5 affects the occurrence and development of many physiopathological processes. Moreover, hAnxA5 can be used independently or in combination as a biomarker of physiopathological phenomena for the diagnosis of certain diseases. Importantly, based on the properties of hAnxA5, many novel drug candidates have been designed and prepared for application in actual medical practice. However, there are also some gaps and shortcomings in hAnxA5 research. This in-depth study will not only expand the understanding of structural and functional relationships but also promote the application of hAnxA5 in the field of biomedicine.
Keywords: human annexin A5; phosphatidylserine (PS); physiopathology; self-assembly.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

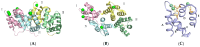
Similar articles
-
Extracellular annexin A5: functions of phosphatidylserine-binding and two-dimensional crystallization.Biochim Biophys Acta. 2008 Jun;1783(6):953-63. doi: 10.1016/j.bbamcr.2008.01.030. Epub 2008 Feb 20. Biochim Biophys Acta. 2008. PMID: 18334229 Review.
-
Lactadherin binding and phosphatidylserine expression on cell surface-comparison with annexin A5.Transl Res. 2006 Jul;148(1):19-25. doi: 10.1016/j.lab.2006.03.006. Transl Res. 2006. PMID: 16887494
-
Cell surface-expressed phosphatidylserine and annexin A5 open a novel portal of cell entry.J Biol Chem. 2004 Dec 10;279(50):52623-9. doi: 10.1074/jbc.M409009200. Epub 2004 Sep 20. J Biol Chem. 2004. PMID: 15381697
-
Annexin A5: shifting from a diagnostic towards a therapeutic realm.Cell Mol Life Sci. 2007 Nov;64(22):2859-62. doi: 10.1007/s00018-007-7297-2. Cell Mol Life Sci. 2007. PMID: 17876516 Free PMC article. Review.
-
Cooperative binding of Annexin A5 to phosphatidylserine on apoptotic cell membranes.Phys Biol. 2013 Dec;10(6):065006. doi: 10.1088/1478-3975/10/6/065006. Epub 2013 Dec 4. Phys Biol. 2013. PMID: 24304966
Cited by
-
Annexin A: Cell Death, Inflammation, and Translational Medicine.J Inflamm Res. 2025 Apr 26;18:5655-5672. doi: 10.2147/JIR.S511439. eCollection 2025. J Inflamm Res. 2025. PMID: 40309306 Free PMC article. Review.
-
Annexin A5 ameliorates H2O2-induced cytotoxicity in SH-SY5Y cells.Mol Biol Rep. 2025 Jun 29;52(1):653. doi: 10.1007/s11033-025-10726-6. Mol Biol Rep. 2025. PMID: 40581889
-
Cancer: A Multifaceted Enemy and the Precision Oncology Response.Int J Mol Sci. 2024 May 21;25(11):5577. doi: 10.3390/ijms25115577. Int J Mol Sci. 2024. PMID: 38891765 Free PMC article.
-
Phosphatidylserine: A Novel Target for Ischemic Stroke Treatment.Biomolecules. 2024 Oct 12;14(10):1293. doi: 10.3390/biom14101293. Biomolecules. 2024. PMID: 39456225 Free PMC article. Review.
-
Beet supplementation mitigates post-exercise inflammation.Front Nutr. 2024 May 30;11:1408804. doi: 10.3389/fnut.2024.1408804. eCollection 2024. Front Nutr. 2024. PMID: 38873567 Free PMC article.
References
-
- Kume K. Isolation of calphobindin-II and its mechanism of anticoagulant activity. Nihon Sanka Fujinka Gakkai Zasshi. 1989;41:1537–1544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

