Retinal Ciliopathies and Potential Gene Therapies: A Focus on Human iPSC-Derived Organoid Models
- PMID: 38474133
- PMCID: PMC10932180
- DOI: 10.3390/ijms25052887
Retinal Ciliopathies and Potential Gene Therapies: A Focus on Human iPSC-Derived Organoid Models
Abstract
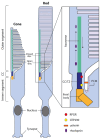
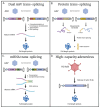
The human photoreceptor function is dependent on a highly specialised cilium. Perturbation of cilial function can often lead to death of the photoreceptor and loss of vision. Retinal ciliopathies are a genetically diverse range of inherited retinal disorders affecting aspects of the photoreceptor cilium. Despite advances in the understanding of retinal ciliopathies utilising animal disease models, they can often lack the ability to accurately mimic the observed patient phenotype, possibly due to structural and functional deviations from the human retina. Human-induced pluripotent stem cells (hiPSCs) can be utilised to generate an alternative disease model, the 3D retinal organoid, which contains all major retinal cell types including photoreceptors complete with cilial structures. These retinal organoids facilitate the study of disease mechanisms and potential therapies in a human-derived system. Three-dimensional retinal organoids are still a developing technology, and despite impressive progress, several limitations remain. This review will discuss the state of hiPSC-derived retinal organoid technology for accurately modelling prominent retinal ciliopathies related to genes, including RPGR, CEP290, MYO7A, and USH2A. Additionally, we will discuss the development of novel gene therapy approaches targeting retinal ciliopathies, including the delivery of large genes and gene-editing techniques.
Keywords: CEP290; CRISPR/Cas9; MYO7A; RPGR; USH2A; adeno-associated virus (AAV); cilium; gene therapy; retinal ciliopathy; retinal organoid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Reserpine maintains photoreceptor survival in retinal ciliopathy by resolving proteostasis imbalance and ciliogenesis defects.Elife. 2023 Mar 28;12:e83205. doi: 10.7554/eLife.83205. Elife. 2023. PMID: 36975211 Free PMC article.
-
Gene Correction Reverses Ciliopathy and Photoreceptor Loss in iPSC-Derived Retinal Organoids from Retinitis Pigmentosa Patients.Stem Cell Reports. 2018 Apr 10;10(4):1267-1281. doi: 10.1016/j.stemcr.2018.02.003. Epub 2018 Mar 8. Stem Cell Reports. 2018. PMID: 29526738 Free PMC article.
-
Using induced pluripotent stem cells to understand retinal ciliopathy disease mechanisms and develop therapies.Biochem Soc Trans. 2016 Oct 15;44(5):1245-1251. doi: 10.1042/BST20160156. Biochem Soc Trans. 2016. PMID: 27911706 Free PMC article. Review.
-
Primary Cilium-Mediated Retinal Pigment Epithelium Maturation Is Disrupted in Ciliopathy Patient Cells.Cell Rep. 2018 Jan 2;22(1):189-205. doi: 10.1016/j.celrep.2017.12.038. Cell Rep. 2018. PMID: 29298421 Free PMC article.
-
Patient stem cell-derived in vitro disease models for developing novel therapies of retinal ciliopathies.Curr Top Dev Biol. 2023;155:127-163. doi: 10.1016/bs.ctdb.2023.09.003. Epub 2023 Nov 4. Curr Top Dev Biol. 2023. PMID: 38043950 Free PMC article. Review.
Cited by
-
The application of retinal organoids in ophthalmic regenerative medicine: A mini-review.Regen Ther. 2024 Jun 30;26:382-386. doi: 10.1016/j.reth.2024.06.013. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39050551 Free PMC article. Review.
-
AAV2.7m8 transduction of stage 2 human retinal organoids induces highly variable responses in innate and inflammatory gene expression and cytokine secretion.Exp Eye Res. 2025 Sep;258:110478. doi: 10.1016/j.exer.2025.110478. Epub 2025 Jun 6. Exp Eye Res. 2025. PMID: 40484363
-
A differential requirement for ciliary transition zone proteins in human and mouse neural progenitor fate specification.Nat Commun. 2025 Apr 5;16(1):3258. doi: 10.1038/s41467-025-58554-3. Nat Commun. 2025. PMID: 40188187 Free PMC article.
-
Retinal Organoids: Innovative Tools for Understanding Retinal Degeneration.Int J Mol Sci. 2025 Apr 1;26(7):3263. doi: 10.3390/ijms26073263. Int J Mol Sci. 2025. PMID: 40244125 Free PMC article. Review.
-
Conventional and Tropism-Modified High-Capacity Adenoviral Vectors Exhibit Similar Transduction Profiles in Human iPSC-Derived Retinal Organoids.Int J Mol Sci. 2024 Dec 24;26(1):55. doi: 10.3390/ijms26010055. Int J Mol Sci. 2024. PMID: 39795914 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

