Monocytes as Targets for Immunomodulation by Regional Citrate Anticoagulation
- PMID: 38474146
- PMCID: PMC10932113
- DOI: 10.3390/ijms25052900
Monocytes as Targets for Immunomodulation by Regional Citrate Anticoagulation
Abstract
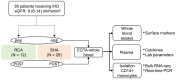
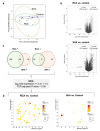
Immune alterations in end-stage renal patients receiving hemodialysis are complex and predispose patients to infections. Anticoagulation may also play an immunomodulatory role in addition to the accumulation of uremic toxins and the effects of the dialysis procedure. Accordingly, it has been recently shown that the infection rate increases in patients under regional citrate anticoagulation (RCA) compared with systemic heparin anticoagulation (SHA). We hypothesized that RCA affects the immune status of hemodialysis patients by targeting monocytes. In a cohort of 38 end-stage renal patients undergoing hemodialysis, we demonstrated that whole blood monocytes of patients receiving RCA-but not SHA-failed to upregulate surface activation markers, like human leukocyte antigen class II (HLA-DR), after stressful insults, indicating a state of deactivation during and immediately after dialysis. Additionally, RNA sequencing (RNA-seq) data and gene set enrichment analysis of pre-dialysis monocytes evidenced a great and complex difference between the groups given that, in the RCA group, monocytes displayed a dramatic transcriptional change with increased expression of genes related to the cell cycle regulation, cellular metabolism, and cytokine signaling, compatible with the reprogramming of the immune response. Transcriptomic changes in pre-dialysis monocytes signalize the lasting nature of the RCA-related effects, suggesting that monocytes are affected even beyond the dialysis session. Furthermore, these findings demonstrate that RCA-but not SHA-impairs the response of monocytes to activation stimuli and alters the immune status of these patients with potential clinical implications.
Keywords: anticoagulation; citrate; hemodialysis; heparin; immunomodulation; monocytes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

