Sindbis Virus Vaccine Platform: A Promising Oncolytic Virus-Mediated Approach for Ovarian Cancer Treatment
- PMID: 38474178
- PMCID: PMC10932354
- DOI: 10.3390/ijms25052925
Sindbis Virus Vaccine Platform: A Promising Oncolytic Virus-Mediated Approach for Ovarian Cancer Treatment
Abstract
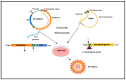
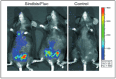
This review article provides a comprehensive overview of a novel Sindbis virus vaccine platform as potential immunotherapy for ovarian cancer patients. Ovarian cancer is the most lethal of all gynecological malignancies. The majority of high-grade serous ovarian cancer (HGSOC) patients are diagnosed with advanced disease. Current treatment options are very aggressive and limited, resulting in tumor recurrences and 50-60% patient mortality within 5 years. The unique properties of armed oncolytic Sindbis virus vectors (SV) in vivo have garnered significant interest in recent years to potently target and treat ovarian cancer. We discuss the molecular biology of Sindbis virus, its mechanisms of action against ovarian cancer cells, preclinical in vivo studies, and future perspectives. The potential of Sindbis virus-based therapies for ovarian cancer treatment holds great promise and warrants further investigation. Investigations using other oncolytic viruses in preclinical studies and clinical trials are also presented.
Keywords: Sindbis virus; immunotherapy; oncolytic virus; ovarian cancer.
Conflict of interest statement
All authors are employed by NYU Langone School of Medicine and have no employment relationship or consultancy agreement with Cynvec, a biotechnology company that supports some studies under a Research and Licensing agreement with NYU. S.O., A.H., C.P., and D.M. are inventors on one or several issued patents and/or patent applications held by NYU that cover the Sindbis treatment of neoplasia and COVID-19. As part of the Research and Licensing agreement, the authors who are inventors on patents are entitled to a portion of the royalties that NYU Langone would receive, should Sindbis vectors be approved by the FDA for therapeutic or vaccination use. Data and materials availability: Correspondence should be addressed to D.M.
Figures


References
-
- National Cancer Institute Surveillance, Epidemiology and End Results Program. [(accessed on 19 September 2022)]; Available online: https://seer.cancer.gov/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

