Thermodynamic and Structural Study of Budesonide-Exogenous Lung Surfactant System
- PMID: 38474237
- PMCID: PMC10931555
- DOI: 10.3390/ijms25052990
Thermodynamic and Structural Study of Budesonide-Exogenous Lung Surfactant System
Abstract
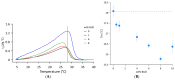
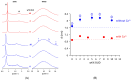
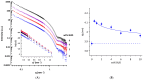
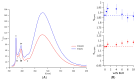
The clinical benefits of using exogenous pulmonary surfactant (EPS) as a carrier of budesonide (BUD), a non-halogenated corticosteroid with a broad anti-inflammatory effect, have been established. Using various experimental techniques (differential scanning calorimetry DSC, small- and wide- angle X-ray scattering SAXS/WAXS, small- angle neutron scattering SANS, fluorescence spectroscopy, dynamic light scattering DLS, and zeta potential), we investigated the effect of BUD on the thermodynamics and structure of the clinically used EPS, Curosurf®. We show that BUD facilitates the Curosurf® phase transition from the gel to the fluid state, resulting in a decrease in the temperature of the main phase transition (Tm) and enthalpy (ΔH). The morphology of the Curosurf® dispersion is maintained for BUD < 10 wt% of the Curosurf® mass; BUD slightly increases the repeat distance d of the fluid lamellar phase in multilamellar vesicles (MLVs) resulting from the thickening of the lipid bilayer. The bilayer thickening (~0.23 nm) was derived from SANS data. The presence of ~2 mmol/L of Ca2+ maintains the effect and structure of the MLVs. The changes in the lateral pressure of the Curosurf® bilayer revealed that the intercalated BUD between the acyl chains of the surfactant's lipid molecules resides deeper in the hydrophobic region when its content exceeds ~6 wt%. Our studies support the concept of a combined therapy utilising budesonide-enriched Curosurf®.
Keywords: SANS; SAXS/WAXS; budesonide; differential scanning calorimetry; lateral pressure; lung surfactant.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Polymyxin B-Enriched Exogenous Lung Surfactant: Thermodynamics and Structure.Langmuir. 2024 Apr 2;40(13):6847-6861. doi: 10.1021/acs.langmuir.3c03746. Epub 2024 Mar 19. Langmuir. 2024. PMID: 38501650
-
Disruptive effect of tocopherol oxalate on DPPC liposome structure: DSC, SAXS, and fluorescence anisotropy studies.Chem Phys Lipids. 2018 Nov;216:104-113. doi: 10.1016/j.chemphyslip.2018.10.001. Epub 2018 Oct 9. Chem Phys Lipids. 2018. PMID: 30308198
-
X-ray structure, thermodynamics, elastic properties and MD simulations of cardiolipin/dimyristoylphosphatidylcholine mixed membranes.Chem Phys Lipids. 2014 Feb;178:1-10. doi: 10.1016/j.chemphyslip.2013.12.010. Epub 2013 Dec 28. Chem Phys Lipids. 2014. PMID: 24378240 Free PMC article.
-
Structure and Thermotropic Behavior of Bovine- and Porcine-Derived Exogenous Lung Surfactants.Langmuir. 2020 Dec 8;36(48):14514-14529. doi: 10.1021/acs.langmuir.0c02224. Epub 2020 Nov 19. Langmuir. 2020. PMID: 33210931
-
Pressure perturbation and differential scanning calorimetric studies of bipolar tetraether liposomes derived from the thermoacidophilic archaeon Sulfolobus acidocaldarius.Biophys J. 2005 Sep;89(3):1841-9. doi: 10.1529/biophysj.105.063933. Epub 2005 Jun 24. Biophys J. 2005. PMID: 15980181 Free PMC article.
Cited by
-
Effects of Nicotine on the Thermodynamics and Phase Coexistence of Pulmonary Surfactant Model Membranes.Membranes (Basel). 2024 Dec 11;14(12):267. doi: 10.3390/membranes14120267. Membranes (Basel). 2024. PMID: 39728717 Free PMC article.
References
-
- Oseliero Filho P.L., Gerbelli B.B., Fornasier F., Chaves Filho A.B., Yoshinaga M.Y., Miyamoto S., Mortara L., Lacerda C.D., Cuccovia I.M., Pimentel A.S., et al. Structure and Thermotropic Behavior of Bovine- And Porcine-Derived Exogenous Lung Surfactants. Langmuir. 2020;36:14514–14529. doi: 10.1021/acs.langmuir.0c02224. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

