Early Pulmonary Fibrosis-like Changes in the Setting of Heat Exposure: DNA Damage and Cell Senescence
- PMID: 38474239
- PMCID: PMC10932106
- DOI: 10.3390/ijms25052992
Early Pulmonary Fibrosis-like Changes in the Setting of Heat Exposure: DNA Damage and Cell Senescence
Abstract
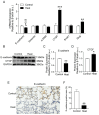
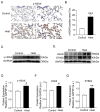
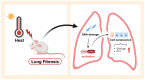
It is well known that extreme heat events happen frequently due to climate change. However, studies examining the direct health impacts of increased temperature and heat waves are lacking. Previous reports revealed that heatstroke induced acute lung injury and pulmonary dysfunction. This study aimed to investigate whether heat exposure induced lung fibrosis and to explore the underlying mechanisms. Male C57BL/6 mice were exposed to an ambient temperature of 39.5 ± 0.5 °C until their core temperature reached the maximum or heat exhaustion state. Lung fibrosis was observed in the lungs of heat-exposed mice, with extensive collagen deposition and the elevated expression of fibrosis molecules, including transforming growth factor-β1 (TGF-β1) and Fibronectin (Fn1) (p < 0.05). Moreover, epithelial-mesenchymal transition (EMT) occurred in response to heat exposure, evidenced by E-cadherin, an epithelial marker, which was downregulated, whereas markers of EMT, such as connective tissue growth factor (CTGF) and the zinc finger transcriptional repressor protein Slug, were upregulated in the heat-exposed lung tissues of mice (p < 0.05). Subsequently, cell senescence examination revealed that the levels of both senescence-associated β-galactosidase (SA-β-gal) staining and the cell cycle protein kinase inhibitor p21 were significantly elevated (p < 0.05). Mechanistically, the cGAS-STING signaling pathway evoked by DNA damage was activated in response to heat exposure (p < 0.05). In summary, we reported a new finding that heat exposure contributed to the development of early pulmonary fibrosis-like changes through the DNA damage-activated cGAS-STING pathway followed by cellular senescence.
Keywords: DNA damage; cGAS–STING pathway; heat exposure; lung fibrosis; senescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Epithelial-mesenchymal transition involved in pulmonary fibrosis induced by multi-walled carbon nanotubes via TGF-beta/Smad signaling pathway.Toxicol Lett. 2014 Apr 21;226(2):150-62. doi: 10.1016/j.toxlet.2014.02.004. Epub 2014 Feb 12. Toxicol Lett. 2014. PMID: 24530353
-
Sulforaphane attenuates pulmonary fibrosis by inhibiting the epithelial-mesenchymal transition.BMC Pharmacol Toxicol. 2018 Apr 2;19(1):13. doi: 10.1186/s40360-018-0204-7. BMC Pharmacol Toxicol. 2018. PMID: 29609658 Free PMC article.
-
Qingfei xieding prescription ameliorates mitochondrial DNA-initiated inflammation in bleomycin-induced pulmonary fibrosis through activating autophagy.J Ethnopharmacol. 2024 May 10;325:117820. doi: 10.1016/j.jep.2024.117820. Epub 2024 Jan 28. J Ethnopharmacol. 2024. PMID: 38286157
-
Hydrogen inhalation attenuated bleomycin-induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial-to-mesenchymal transition.Exp Physiol. 2019 Dec;104(12):1942-1951. doi: 10.1113/EP088028. Epub 2019 Oct 23. Exp Physiol. 2019. PMID: 31535412
-
Cytokines as drivers: Unraveling the mechanisms of epithelial-mesenchymal transition in COVID-19 lung fibrosis.Biochem Biophys Res Commun. 2023 Dec 17;686:149118. doi: 10.1016/j.bbrc.2023.10.050. Epub 2023 Oct 14. Biochem Biophys Res Commun. 2023. PMID: 37931361 Review.
Cited by
-
Pulmonary vascular disease, environmental pollution, and climate change.Pulm Circ. 2024 Jun 26;14(2):e12394. doi: 10.1002/pul2.12394. eCollection 2024 Apr. Pulm Circ. 2024. PMID: 38933180 Free PMC article. Review.
-
Regulation of cellular senescence in tumor progression and therapeutic targeting: mechanisms and pathways.Mol Cancer. 2025 Apr 2;24(1):106. doi: 10.1186/s12943-025-02284-z. Mol Cancer. 2025. PMID: 40170077 Free PMC article. Review.
-
COPD risk due to extreme temperature exposure: combining epidemiological evidence with pathophysiological mechanisms.EBioMedicine. 2025 Jun;116:105731. doi: 10.1016/j.ebiom.2025.105731. Epub 2025 Apr 30. EBioMedicine. 2025. PMID: 40311422 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

