Emerging Role of GCN1 in Disease and Homeostasis
- PMID: 38474243
- PMCID: PMC10931611
- DOI: 10.3390/ijms25052998
Emerging Role of GCN1 in Disease and Homeostasis
Abstract
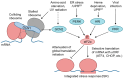
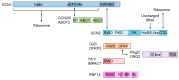
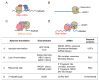
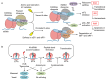
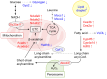
GCN1 is recognized as a factor that is essential for the activation of GCN2, which is a sensor of amino acid starvation. This function is evolutionarily conserved from yeast to higher eukaryotes. However, recent studies have revealed non-canonical functions of GCN1 that are independent of GCN2, such as its participation in cell proliferation, apoptosis, and the immune response, beyond the borders of species. Although it is known that GCN1 and GCN2 interact with ribosomes to accomplish amino acid starvation sensing, recent studies have reported that GCN1 binds to disomes (i.e., ribosomes that collide each other), thereby regulating both the co-translational quality control and stress response. We propose that GCN1 regulates ribosome-mediated signaling by dynamically changing its partners among RWD domain-possessing proteins via unknown mechanisms. We recently demonstrated that GCN1 is essential for cell proliferation and whole-body energy regulation in mice. However, the manner in which ribosome-initiated signaling via GCN1 is related to various physiological functions warrants clarification. GCN1-mediated mechanisms and its interaction with other quality control and stress response signals should be important for proteostasis during aging and neurodegenerative diseases, and may be targeted for drug development.
Keywords: GCN1; GCN2; RWD domain; amino acid starvation; disome; ribosomal stress surveillance; ribosome.
Conflict of interest statement
Author Daichi Kokubu is employed by the company KAGOME, Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

