Changes in Inflammatory Cytokines in Responders and Non-Responders to TNFα Inhibitor and IL-17A Inhibitor: A Study Examining Psoriatic Arthritis Patients
- PMID: 38474247
- PMCID: PMC10932211
- DOI: 10.3390/ijms25053002
Changes in Inflammatory Cytokines in Responders and Non-Responders to TNFα Inhibitor and IL-17A Inhibitor: A Study Examining Psoriatic Arthritis Patients
Abstract
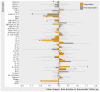
This study aimed to examine the changes in biomarker levels in responders and non-responders to tumor necrosis factor alpha inhibitor (TNFi) and interleukin-17A inhibitor (IL-17Ai) in psoriatic arthritis (PsA) patients over a 4-month period after treatment initiation. A total of 68 PsA patients initiating either TNFi, IL-17Ai, or methotrexate treatment were included. Blood plasma and clinical outcome measures were collected adjacent to treatment initiation and after four months. A commercially available multiplex immunoassay was included to evaluate 54 biomarkers. Mean changes were used to evaluate change over time. A statistically significant decrease in pro-inflammatory cytokines IL-6 (log-transformed mean change -0.97, 95%CI -4.30; 2.37, [p = 0.032]) and an increase in anti-inflammatory IL-10 (0.38, 95%CI 1.74; 2.50 [p = 0.010]) were seen in TNFi responders. Meanwhile, a statistically significant increase in the target cytokine IL-17A was seen in both IL-17Ai responders (2.49, 95%CI -1.84; 6.85 [p = 0.031]) and non-responders (2.48, 95%CI -1.46; 6.41 [p = 0.001]). This study demonstrated differing changes in cytokine levels when comparing treatment responders and non-responders, highlighting the need to improve the understanding of the different immune response mechanisms explaining different responses to medical treatment in PsA patients.
Keywords: IL-17A; TNFα; bDMARDs; cytokine; psoriatic arthritis.
Conflict of interest statement
Marie Skougaard has received research funding from the Danish Rheumatism Association, the Danish National Psoriasis Foundation, Lilly and Pfizer, and a speaker fee from Janssen-Cilag. Sisse B. Ditlev has no conflicts of interest. Magnus Friis Søndergaard has no conflicts of interest. Lars Erik Kristensen has received fees for speaking and consultancy from AbbVie, Amgen, Biogen, Bristol Myers Squibb, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer, and UCB, as well as IIT grants from Biogen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Skougaard M., Jørgensen T.S., Jensen M.J., Ballegaard C., Guldberg-Møller J., Egeberg A., Christensen R., Benzin P., Stisen Z.R., Merola J.F., et al. Change in psoriatic arthritis outcome measures impacts SF-36 physical and mental component score differently: An observational cohort study. Rheumatol. Adv. Pract. 2021;5:rkab076. doi: 10.1093/rap/rkab076. - DOI - PMC - PubMed
-
- Kristensen L.E., Lie E., Jacobsson L.T., Christensen R., Mease P.J., Bliddal H., Geborek P. Effectiveness and feasibility associated with switching to a second or third TNF inhibitor in patients with psoriatic arthritis: A cohort study from southern Sweden. J. Rheumatol. 2016;43:81–87. doi: 10.3899/jrheum.150744. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

