Sphingolipid-Induced Bone Regulation and Its Emerging Role in Dysfunction Due to Disease and Infection
- PMID: 38474268
- PMCID: PMC10932382
- DOI: 10.3390/ijms25053024
Sphingolipid-Induced Bone Regulation and Its Emerging Role in Dysfunction Due to Disease and Infection
Abstract
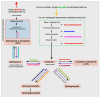
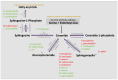
The human skeleton is a metabolically active system that is constantly regenerating via the tightly regulated and highly coordinated processes of bone resorption and formation. Emerging evidence reveals fascinating new insights into the role of sphingolipids, including sphingomyelin, sphingosine, ceramide, and sphingosine-1-phosphate, in bone homeostasis. Sphingolipids are a major class of highly bioactive lipids able to activate distinct protein targets including, lipases, phosphatases, and kinases, thereby conferring distinct cellular functions beyond energy metabolism. Lipids are known to contribute to the progression of chronic inflammation, and notably, an increase in bone marrow adiposity parallel to elevated bone loss is observed in most pathological bone conditions, including aging, rheumatoid arthritis, osteoarthritis, and osteomyelitis. Of the numerous classes of lipids that form, sphingolipids are considered among the most deleterious. This review highlights the important primary role of sphingolipids in bone homeostasis and how dysregulation of these bioactive metabolites appears central to many chronic bone-related diseases. Further, their contribution to the invasion, virulence, and colonization of both viral and bacterial host cell infections is also discussed. Many unmet clinical needs remain, and data to date suggest the future use of sphingolipid-targeted therapy to regulate bone dysfunction due to a variety of diseases or infection are highly promising. However, deciphering the biochemical and molecular mechanisms of this diverse and extremely complex sphingolipidome, both in terms of bone health and disease, is considered the next frontier in the field.
Keywords: bone; disease; infection; osteoporosis; sphingolipids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Role of bioactive sphingolipids in physiology and pathology.Essays Biochem. 2020 Sep 23;64(3):579-589. doi: 10.1042/EBC20190091. Essays Biochem. 2020. PMID: 32579188 Review.
-
Role of Canonical and Non-Canonical Sphingolipids and their Metabolic Enzymes in Bone Health.Curr Osteoporos Rep. 2025 Apr 23;23(1):21. doi: 10.1007/s11914-025-00908-3. Curr Osteoporos Rep. 2025. PMID: 40266422 Free PMC article. Review.
-
Nuclear sphingolipid metabolism.Annu Rev Physiol. 2012;74:131-51. doi: 10.1146/annurev-physiol-020911-153321. Epub 2011 Sep 9. Annu Rev Physiol. 2012. PMID: 21888508 Free PMC article. Review.
-
[The role of sphingolipids in selected cardiovascular diseases].Postepy Hig Med Dosw (Online). 2013 Sep 30;67:1018-26. doi: 10.5604/17322693.1068694. Postepy Hig Med Dosw (Online). 2013. PMID: 24088546 Review. Polish.
-
Solving the enigma: Mass spectrometry and small molecule probes to study sphingolipid function.Curr Opin Chem Biol. 2021 Dec;65:49-56. doi: 10.1016/j.cbpa.2021.05.001. Epub 2021 Jun 25. Curr Opin Chem Biol. 2021. PMID: 34175552 Review.
Cited by
-
Ultra-High-Performance Liquid Chromatography-High-Definition Mass Spectrometry-Based Metabolomics to Reveal the Potential Anti-Arthritic Effects of Illicium verum in Cultured Fibroblast-like Synoviocytes Derived from Rheumatoid Arthritis.Metabolites. 2024 Sep 25;14(10):517. doi: 10.3390/metabo14100517. Metabolites. 2024. PMID: 39452898 Free PMC article.
-
Ceramides-Emerging Biomarkers of Lipotoxicity in Obesity, Diabetes, Cardiovascular Diseases, and Inflammation.Diseases. 2024 Aug 23;12(9):195. doi: 10.3390/diseases12090195. Diseases. 2024. PMID: 39329864 Free PMC article. Review.
-
A new perspective in avian bone health: dietary supplementation with a standardized dry grape extract improves pullets' bones' quality through metabolic modulation.Poult Sci. 2025 Aug;104(8):105270. doi: 10.1016/j.psj.2025.105270. Epub 2025 May 7. Poult Sci. 2025. PMID: 40418877 Free PMC article.
-
Altered metabolomics and inflammatory transcriptomics in human bone marrow adipocytes after acute high calorie diet and acute fasting.Front Endocrinol (Lausanne). 2025 Jun 16;16:1591280. doi: 10.3389/fendo.2025.1591280. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40589518 Free PMC article.
-
From Genomics to Metabolomics: Molecular Insights into Osteoporosis for Enhanced Diagnostic and Therapeutic Approaches.Biomedicines. 2024 Oct 18;12(10):2389. doi: 10.3390/biomedicines12102389. Biomedicines. 2024. PMID: 39457701 Free PMC article. Review.
References
-
- Liebisch G., Fahy E., Aoki J., Dennis E.A., Durand T., Ejsing C.S., Fedorova M., Feussner I., Griffiths W.J., Köfeler H., et al. Update on LIPID MAPS Classification, Nomenclature, and Shorthand Notation for MS-Derived Lipid Structures. J. Lipid Res. 2020;61:1539–1555. doi: 10.1194/jlr.S120001025. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

