Tissue Hypoxia and Associated Innate Immune Factors in Experimental Autoimmune Optic Neuritis
- PMID: 38474322
- PMCID: PMC10932468
- DOI: 10.3390/ijms25053077
Tissue Hypoxia and Associated Innate Immune Factors in Experimental Autoimmune Optic Neuritis
Abstract
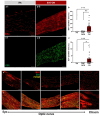
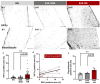
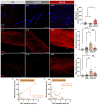
Visual loss in acute optic neuritis is typically attributed to axonal conduction block due to inflammatory demyelination, but the mechanisms remain unclear. Recent research has highlighted tissue hypoxia as an important cause of neurological deficits and tissue damage in both multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) and, here, we examine whether the optic nerves are hypoxic in experimental optic neuritis induced in Dark Agouti rats. At both the first and second peaks of disease expression, inflamed optic nerves labelled significantly for tissue hypoxia (namely, positive for hypoxia inducible factor-1α (HIF1α) and intravenously administered pimonidazole). Acutely inflamed nerves were also labelled significantly for innate markers of oxidative and nitrative stress and damage, including superoxide, nitric oxide and 3-nitrotyrosine. The density and diameter of capillaries were also increased. We conclude that in acute optic neuritis, the optic nerves are hypoxic and come under oxidative and nitrative stress and damage. Tissue hypoxia can cause mitochondrial failure and thus explains visual loss due to axonal conduction block. Tissue hypoxia can also induce a damaging oxidative and nitrative environment. The findings indicate that treatment to prevent tissue hypoxia in acute optic neuritis may help to restore vision and protect from damaging reactive oxygen and nitrogen species.
Keywords: hypoxia inducible factor-1α; multiple sclerosis; nitric oxide; oxidative stress; peroxynitrite; superoxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Loss of Nrf2 exacerbates the visual deficits and optic neuritis elicited by experimental autoimmune encephalomyelitis.Mol Vis. 2016 Dec 30;22:1503-1513. eCollection 2016. Mol Vis. 2016. PMID: 28050123 Free PMC article.
-
Elevation of AQP4 and selective cytokines in experimental autoimmune encephalitis mice provides some potential biomarkers in optic neuritis and demyelinating diseases.Int J Clin Exp Pathol. 2015 Dec 1;8(12):15749-58. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26884844 Free PMC article.
-
Non-invasive visual evoked potentials to assess optic nerve involvement in the dark agouti rat model of experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein.Brain Pathol. 2020 Jan;30(1):137-150. doi: 10.1111/bpa.12762. Epub 2019 Jul 28. Brain Pathol. 2020. PMID: 31267597 Free PMC article.
-
A putative role for calpain in demyelination associated with optic neuritis.Histol Histopathol. 1999 Apr;14(2):649-56. doi: 10.14670/HH-14.649. Histol Histopathol. 1999. PMID: 10212825 Review.
-
Assessing the anterior visual pathway in optic neuritis: recent experimental and clinical aspects.Curr Opin Neurol. 2019 Jun;32(3):346-357. doi: 10.1097/WCO.0000000000000675. Curr Opin Neurol. 2019. PMID: 30694926 Review.
Cited by
-
A Hypoxia-Inflammation Cycle and Multiple Sclerosis: Mechanisms and Therapeutic Implications.Curr Treat Options Neurol. 2025;27(1):6. doi: 10.1007/s11940-024-00816-4. Epub 2024 Nov 18. Curr Treat Options Neurol. 2025. PMID: 39569339 Free PMC article. Review.
-
Biomolecular Dynamics of Nitric Oxide Metabolites and HIF1α in HPV Infection.Biomolecules. 2024 Sep 18;14(9):1172. doi: 10.3390/biom14091172. Biomolecules. 2024. PMID: 39334938 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

