Red Blood Cell Storage with Xenon: Safe or Disruption?
- PMID: 38474375
- PMCID: PMC10930635
- DOI: 10.3390/cells13050411
Red Blood Cell Storage with Xenon: Safe or Disruption?
Abstract
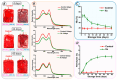
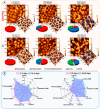
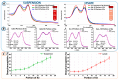
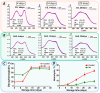
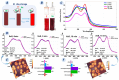
Xenon, an inert gas commonly used in medicine, has been considered as a potential option for prolonged preservation of donor packed red blood cells (pRBCs) under hypoxic conditions. This study aimed to investigate how xenon affects erythrocyte parameters under prolonged storage. In vitro model experiments were performed using two methods to create hypoxic conditions. In the first method, xenon was introduced into bags of pRBCs which were then stored for 42 days, while in the second method, xenon was added to samples in glass tubes. The results of our experiment showed that the presence of xenon resulted in notable alterations in erythrocyte morphology, similar to those observed under standard storage conditions. For pRBC bags, hemolysis during storage with xenon exceeded the acceptable limit by a factor of six, whereas the closed-glass-tube experiment showed minimal hemolysis in samples exposed to xenon. Notably, the production of deoxyhemoglobin was specific to xenon exposure in both cell suspension and hemolysate. However, this study did not provide evidence for the purported protective properties of xenon.
Keywords: AFM; blood storage; deoxyhemoglobin; in vitro study; membrane; red blood cells; spectrophotometry; xenon.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Moroz V.V., Sherstyukova E.A., Kozlova E.K., Sergunova V.A. Storage Time of Filtered Red Blood Cells and Post-Transfusion Complications (Review) Gen. Reanimatol. 2021;17:69–82. doi: 10.15360/1813-9779-2021-1-69-82. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

