The Use of Immune Regulation in Treating Head and Neck Squamous Cell Carcinoma (HNSCC)
- PMID: 38474377
- PMCID: PMC10930979
- DOI: 10.3390/cells13050413
The Use of Immune Regulation in Treating Head and Neck Squamous Cell Carcinoma (HNSCC)
Abstract
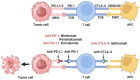
Immunotherapy has emerged as a promising new treatment modality for head and neck cancer, offering the potential for targeted and effective cancer management. Squamous cell carcinomas pose significant challenges due to their aggressive nature and limited treatment options. Conventional therapies such as surgery, radiation, and chemotherapy often have limited success rates and can have significant side effects. Immunotherapy harnesses the power of the immune system to recognize and eliminate cancer cells, and thus represents a novel approach with the potential to improve patient outcomes. In the management of head and neck squamous cell carcinoma (HNSCC), important contributions are made by immunotherapies, including adaptive cell therapy (ACT) and immune checkpoint inhibitor therapy. In this review, we are focusing on the latter. Immune checkpoint inhibitors target proteins such as programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) to enhance the immune response against cancer cells. The CTLA-4 inhibitors, such as ipilimumab and tremelimumab, have been approved for early-stage clinical trials and have shown promising outcomes in terms of tumor regression and durable responses in patients with advanced HNSCC. Thus, immune checkpoint inhibitor therapy holds promise in overcoming the limitations of conventional therapies. However, further research is needed to optimize treatment regimens, identify predictive biomarkers, and overcome potential resistance mechanisms. With ongoing advancements in immunotherapy, the future holds great potential for transforming the landscape of oral tumor treatment and providing new hope for patients.
Keywords: combination therapy; cytotoxic T-lymphocyte-associated protein 4 (CTLA-4); head and neck squamous cell carcinoma (HNSCC); immune checkpoint inhibitors; immunotherapy; oral carcinoma; programmed cell death protein 1; programmed death ligand-1 (PD-1/PD-L1); tumor microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Immune checkpoint inhibitors in head and neck squamous cell carcinoma: A systematic review of phase-3 clinical trials.World J Clin Oncol. 2022 May 24;13(5):388-411. doi: 10.5306/wjco.v13.i5.388. World J Clin Oncol. 2022. PMID: 35662989 Free PMC article.
-
The dynamic role of immune checkpoint molecules in diagnosis, prognosis, and treatment of head and neck cancers.Biomed Pharmacother. 2024 Feb;171:116095. doi: 10.1016/j.biopha.2023.116095. Epub 2024 Jan 6. Biomed Pharmacother. 2024. PMID: 38183744 Review.
-
The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC).J Immunother Cancer. 2019 Jul 15;7(1):184. doi: 10.1186/s40425-019-0662-5. J Immunother Cancer. 2019. PMID: 31307547 Free PMC article. Clinical Trial.
-
Initial analysis of the synergy of programmed cell death-1 (PD-1) inhibitor and concurrent chemoradiotherapy treatment for recurrent/metastatic head and neck squamous cell carcinoma patients.Radiat Oncol. 2023 Jul 4;18(1):109. doi: 10.1186/s13014-023-02310-8. Radiat Oncol. 2023. PMID: 37403098 Free PMC article.
-
The effects of checkpoint inhibition on head and neck squamous cell carcinoma: A systematic review.Oral Oncol. 2019 Mar;90:67-73. doi: 10.1016/j.oraloncology.2019.01.018. Epub 2019 Feb 5. Oral Oncol. 2019. PMID: 30846179
Cited by
-
Immune Evasion in Head and Neck Squamous Cell Carcinoma: Roles of Cancer-Associated Fibroblasts, Immune Checkpoints, and TP53 Mutations in the Tumor Microenvironment.Cancers (Basel). 2025 Aug 7;17(15):2590. doi: 10.3390/cancers17152590. Cancers (Basel). 2025. PMID: 40805285 Free PMC article. Review.
-
Advances in neoantigen-based immunotherapy for head and neck squamous cell carcinoma: a comprehensive review.Front Oncol. 2025 May 15;15:1593048. doi: 10.3389/fonc.2025.1593048. eCollection 2025. Front Oncol. 2025. PMID: 40444094 Free PMC article. Review.
-
Dendritic cell-related hub genes in head-and-neck squamous cell carcinoma: implications for prognosis and immunotherapy.Transl Cancer Res. 2024 Jul 31;13(7):3620-3636. doi: 10.21037/tcr-23-2360. Epub 2024 Jul 22. Transl Cancer Res. 2024. PMID: 39145060 Free PMC article.
-
Severe immunotherapy-related thrombocytopenia in metastatic bone cancer: a multicenter retrospective case series highlighting early recognition and management.Front Oncol. 2025 May 27;15:1574379. doi: 10.3389/fonc.2025.1574379. eCollection 2025. Front Oncol. 2025. PMID: 40496609 Free PMC article.
-
CircSLC22A3 inhibits the invasion and metastasis of ESCC via the miR-19b-3p/TRAK2 axis and by reducing the stability of m6A-modified ACSBG1 mRNA.BMC Cancer. 2025 May 30;25(1):971. doi: 10.1186/s12885-025-14390-8. BMC Cancer. 2025. PMID: 40448098 Free PMC article.
References
-
- Sloan P., Gale N., Hunter K., Lingen M., Nylander K., Reibel J. Tumours of the oral cavity and mobile tongue: Malignant surface epithelial tumors: Squamous cell carcinoma. WHO Classif. Head Neck Tumours. 2017;9:108–109.
-
- De Virgilio A., Costantino A., Mercante G., Petruzzi G., Sebastiani D., Franzese C., Scorsetti M., Pellini R., Malvezzi L., Spriano G. Present and Future of De-intensification Strategies in the Treatment of Oropharyngeal Carcinoma. Curr. Oncol. Rep. 2020;22:91. doi: 10.1007/s11912-020-00948-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

