Hedgehog on the Move: Glypican-Regulated Transport and Gradient Formation in Drosophila
- PMID: 38474382
- PMCID: PMC10930589
- DOI: 10.3390/cells13050418
Hedgehog on the Move: Glypican-Regulated Transport and Gradient Formation in Drosophila
Abstract
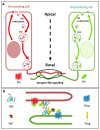
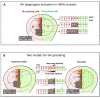
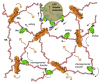
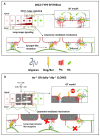
Glypicans (Glps) are a family of heparan sulphate proteoglycans that are attached to the outer plasma membrane leaflet of the producing cell by a glycosylphosphatidylinositol anchor. Glps are involved in the regulation of many signalling pathways, including those that regulate the activities of Wnts, Hedgehog (Hh), Fibroblast Growth Factors (FGFs), and Bone Morphogenetic Proteins (BMPs), among others. In the Hh-signalling pathway, Glps have been shown to be essential for ligand transport and the formation of Hh gradients over long distances, for the maintenance of Hh levels in the extracellular matrix, and for unimpaired ligand reception in distant recipient cells. Recently, two mechanistic models have been proposed to explain how Hh can form the signalling gradient and how Glps may contribute to it. In this review, we describe the structure, biochemistry, and metabolism of Glps and their interactions with different components of the Hh-signalling pathway that are important for the release, transport, and reception of Hh.
Keywords: Dally; Dally like; Hedgehog; glypicans; heparan sulphate proteoglycans.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process.Development. 2004 Feb;131(3):601-11. doi: 10.1242/dev.00958. Epub 2004 Jan 7. Development. 2004. PMID: 14729575
-
Dally-like core protein and its mammalian homologues mediate stimulatory and inhibitory effects on Hedgehog signal response.Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):5869-74. doi: 10.1073/pnas.1001777107. Epub 2010 Mar 15. Proc Natl Acad Sci U S A. 2010. PMID: 20231458 Free PMC article.
-
The Drosophila WIF1 homolog Shifted maintains glypican-independent Hedgehog signaling and interacts with the Hedgehog co-receptors Ihog and Boi.Development. 2013 Jan 1;140(1):107-16. doi: 10.1242/dev.078444. Epub 2012 Nov 15. Development. 2013. PMID: 23154411 Free PMC article.
-
Glypican-mediated endocytosis of Hedgehog has opposite effects in flies and mice.Trends Cell Biol. 2008 Aug;18(8):360-3. doi: 10.1016/j.tcb.2008.06.001. Epub 2008 Jul 5. Trends Cell Biol. 2008. PMID: 18603427 Review.
-
The role of glypicans in Hedgehog signaling.Matrix Biol. 2014 Apr;35:248-52. doi: 10.1016/j.matbio.2013.12.007. Epub 2014 Jan 8. Matrix Biol. 2014. PMID: 24412155 Review.
Cited by
-
Single-cell sequencing suggests a conserved function of Hedgehog-signalling in spider eye development.Evodevo. 2024 Sep 26;15(1):11. doi: 10.1186/s13227-024-00230-6. Evodevo. 2024. PMID: 39327634 Free PMC article.
References
-
- Bilioni A., Sánchez-Hernández D., Callejo A., Gradilla A.-C., Ibáñez C., Mollica E., Rodríguez-Navas M.C., Simon E., Guerrero I. Balancing Hedgehog, a retention and release equilibrium given by Dally, Ihog, Boi and shifted/DmWif. Dev. Biol. 2013;376:198–212. doi: 10.1016/j.ydbio.2012.12.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

