Role of Inflammatory Mechanisms in Major Depressive Disorder: From Etiology to Potential Pharmacological Targets
- PMID: 38474387
- PMCID: PMC10931285
- DOI: 10.3390/cells13050423
Role of Inflammatory Mechanisms in Major Depressive Disorder: From Etiology to Potential Pharmacological Targets
Abstract
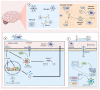
The involvement of central and peripheral inflammation in the pathogenesis and prognosis of major depressive disorder (MDD) has been demonstrated. The increase of pro-inflammatory cytokines (interleukin (IL)-1β, IL-6, IL-18, and TNF-α) in individuals with depression may elicit neuroinflammatory processes and peripheral inflammation, mechanisms that, in turn, can contribute to gut microbiota dysbiosis. Together, neuroinflammation and gut dysbiosis induce alterations in tryptophan metabolism, culminating in decreased serotonin synthesis, impairments in neuroplasticity-related mechanisms, and glutamate-mediated excitotoxicity. This review aims to highlight the inflammatory mechanisms (neuroinflammation, peripheral inflammation, and gut dysbiosis) involved in the pathophysiology of MDD and to explore novel anti-inflammatory therapeutic approaches for this psychiatric disturbance. Several lines of evidence have indicated that in addition to antidepressants, physical exercise, probiotics, and nutraceuticals (agmatine, ascorbic acid, and vitamin D) possess anti-inflammatory effects that may contribute to their antidepressant properties. Further studies are necessary to explore the therapeutic benefits of these alternative therapies for MDD.
Keywords: anti-inflammatory approaches; gut dysbiosis; inflammation; major depressive disorder.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association; Washington, DC, USA: 2013. DSM-5 Task Force.
-
- World Health Organization Depressive Disorder (Depression) 2023. [(accessed on 12 January 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/depression.
-
- Caldiroli A., Capuzzi E., Tagliabue I., Capellazzi M., Marcatili M., Mucci F., Colmegna F., Clerici M., Buoli M., Dakanalis A. Augmentative Pharmacological Strategies in Treatment-Resistant Major Depression: A Comprehensive Review. Int. J. Mol. Sci. 2021;22:13070. doi: 10.3390/ijms222313070. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CNPq; #312215/2021-5/National Council for Scientific and Technological Development
- N/A/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- N/A/Fundação de Amparo à Pesquisa e Inovação de Santa Catarina
- UVic-FAPESP SPRINT 1/2018/University of Victoria (UVic, Victoria, BC, Canada) - São Paulo Research FPundation (FAPESP, São Paulo, SP, Brazil) SPRINT partnership
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

