Lung Cancers: Parenchymal Biochemistry and Mechanics
- PMID: 38474391
- PMCID: PMC10931145
- DOI: 10.3390/cells13050427
Lung Cancers: Parenchymal Biochemistry and Mechanics
Abstract
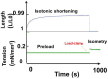
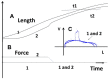
Parenchyma of pulmonary cancers acquires contractile properties that resemble those of muscles but presents some particularities. These non-muscle contractile tissues could be stimulated either electrically or chemically (KCl). They present the Frank-Starling mechanism, the Hill hyperbolic tension-velocity relationship, and the tridimensional time-independent tension-velocity-length relationship. Relaxation could be obtained by the inhibition of crossbridge molecular motors or by a decrease in the intracellular calcium concentration. They differ from muscles in that their kinetics are ultraslow as evidenced by their low shortening velocity and myosin ATPase activity. Contractility is generated by non-muscle myosin type II A and II B. The activation of the β-catenin/WNT pathway is accompanied by the high level of the non-muscle myosin observed in lung cancers.
Keywords: canonical WNT pathway; lung cancer; mechanics; myofibroblast; non-muscle myosin; β-catenin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Frank O. Zur Dynamik des Herzmuskels. Z. Biol. 1895;32:273–277.
-
- Starling E.H. The Arris and Gale Lectures on some points in the pathology of heart disease. Lecture I. The compensating mechanism of the heart. Lancet. 1897;1:569–572. doi: 10.1016/S0140-6736(00)64622-6. - DOI
-
- Hill A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. Biol. Sci. 1938;126:136–195. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

