Exploring Extracellular Matrix Crosslinking as a Therapeutic Approach to Fibrosis
- PMID: 38474402
- PMCID: PMC10931134
- DOI: 10.3390/cells13050438
Exploring Extracellular Matrix Crosslinking as a Therapeutic Approach to Fibrosis
Abstract
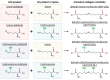
The extracellular matrix (ECM) provides structural support for tissues and regulatory signals for resident cells. ECM requires a careful balance between protein accumulation and degradation for homeostasis. Disruption of this balance can lead to pathological processes such as fibrosis in organs across the body. Post-translational crosslinking modifications to ECM proteins such as collagens alter ECM structure and function. Dysregulation of crosslinking enzymes as well as changes in crosslinking composition are prevalent in fibrosis. Because of the crucial roles these ECM crosslinking pathways play in disease, the enzymes that govern crosslinking events are being explored as therapeutic targets for fibrosis. Here, we review in depth the molecular mechanisms underlying ECM crosslinking, how ECM crosslinking contributes to fibrosis, and the therapeutic strategies being explored to target ECM crosslinking in fibrosis to restore normal tissue structure and function.
Keywords: collagen; crosslinking; extracellular matrix; fibrosis.
Conflict of interest statement
S.M.L. and Y.H. are employees of AbbVie. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

