Advances and Challenges in Sepsis Management: Modern Tools and Future Directions
- PMID: 38474403
- PMCID: PMC10931424
- DOI: 10.3390/cells13050439
Advances and Challenges in Sepsis Management: Modern Tools and Future Directions
Abstract
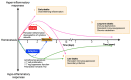
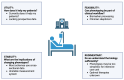
Sepsis, a critical condition marked by systemic inflammation, profoundly impacts both innate and adaptive immunity, often resulting in lymphopenia. This immune alteration can spare regulatory T cells (Tregs) but significantly affects other lymphocyte subsets, leading to diminished effector functions, altered cytokine profiles, and metabolic changes. The complexity of sepsis stems not only from its pathophysiology but also from the heterogeneity of patient responses, posing significant challenges in developing universally effective therapies. This review emphasizes the importance of phenotyping in sepsis to enhance patient-specific diagnostic and therapeutic strategies. Phenotyping immune cells, which categorizes patients based on clinical and immunological characteristics, is pivotal for tailoring treatment approaches. Flow cytometry emerges as a crucial tool in this endeavor, offering rapid, low cost and detailed analysis of immune cell populations and their functional states. Indeed, this technology facilitates the understanding of immune dysfunctions in sepsis and contributes to the identification of novel biomarkers. Our review underscores the potential of integrating flow cytometry with omics data, machine learning and clinical observations to refine sepsis management, highlighting the shift towards personalized medicine in critical care. This approach could lead to more precise interventions, improving outcomes in this heterogeneously affected patient population.
Keywords: biomarkers; flow cytometry; immune response; lymphopenia; personalized medicine; phenotyping; sepsis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- de Grooth H.-J., Postema J., Loer S.A., Parienti J.-J., Oudemans-van Straaten H.M., Girbes A.R. Unexplained Mortality Differences between Septic Shock Trials: A Systematic Analysis of Population Characteristics and Control-Group Mortality Rates. Intensive Care Med. 2018;44:311–322. doi: 10.1007/s00134-018-5134-8. - DOI - PMC - PubMed
-
- Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions—World|ReliefWeb. [(accessed on 9 January 2024)]. Available online: https://reliefweb.int/report/world/global-report-epidemiology-and-burden....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

