Emerging Role of Autophagy in Governing Cellular Dormancy, Metabolic Functions, and Therapeutic Responses of Cancer Stem Cells
- PMID: 38474411
- PMCID: PMC10930960
- DOI: 10.3390/cells13050447
Emerging Role of Autophagy in Governing Cellular Dormancy, Metabolic Functions, and Therapeutic Responses of Cancer Stem Cells
Abstract
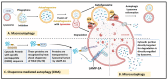
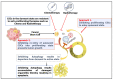
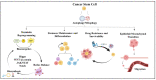
Tumors are composed of heterogeneous populations of dysregulated cells that grow in specialized niches that support their growth and maintain their properties. Tumor heterogeneity and metastasis are among the major hindrances that exist while treating cancer patients, leading to poor clinical outcomes. Although the factors that determine tumor complexity remain largely unknown, several genotypic and phenotypic changes, including DNA mutations and metabolic reprograming provide cancer cells with a survival advantage over host cells and resistance to therapeutics. Furthermore, the presence of a specific population of cells within the tumor mass, commonly known as cancer stem cells (CSCs), is thought to initiate tumor formation, maintenance, resistance, and recurrence. Therefore, these CSCs have been investigated in detail recently as potential targets to treat cancer and prevent recurrence. Understanding the molecular mechanisms involved in CSC proliferation, self-renewal, and dormancy may provide important clues for developing effective therapeutic strategies. Autophagy, a catabolic process, has long been recognized to regulate various physiological and pathological processes. In addition to regulating cancer cells, recent studies have identified a critical role for autophagy in regulating CSC functions. Autophagy is activated under various adverse conditions and promotes cellular maintenance, survival, and even cell death. Thus, it is intriguing to address whether autophagy promotes or inhibits CSC functions and whether autophagy modulation can be used to regulate CSC functions, either alone or in combination. This review describes the roles of autophagy in the regulation of metabolic functions, proliferation and quiescence of CSCs, and its role during therapeutic stress. The review further highlights the autophagy-associated pathways that could be used to regulate CSCs. Overall, the present review will help to rationalize various translational approaches that involve autophagy-mediated modulation of CSCs in controlling cancer progression, metastasis, and recurrence.
Keywords: autophagy; cancer stem cells; chemotherapy; metabolic functions; mitophagy; quiescence; radiotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Targeting redox regulation and autophagy systems in cancer stem cells.Clin Exp Med. 2023 Sep;23(5):1405-1423. doi: 10.1007/s10238-022-00955-5. Epub 2022 Dec 6. Clin Exp Med. 2023. PMID: 36473988 Review.
-
Tumor dormancy and cancer stem cells: two sides of the same coin?Adv Exp Med Biol. 2013;734:145-79. doi: 10.1007/978-1-4614-1445-2_8. Adv Exp Med Biol. 2013. PMID: 23143979
-
Crosstalk between autophagy and CSCs: molecular mechanisms and translational implications.Cell Death Dis. 2023 Jul 8;14(7):409. doi: 10.1038/s41419-023-05929-3. Cell Death Dis. 2023. PMID: 37422448 Free PMC article. Review.
-
Resistance to Cell Death and Its Modulation in Cancer Stem Cells.Crit Rev Oncog. 2016;21(3-4):203-219. doi: 10.1615/CritRevOncog.2016016976. Crit Rev Oncog. 2016. PMID: 27915972 Free PMC article. Review.
-
Cancer Stem Cells and Their Therapeutic Usage.Adv Exp Med Biol. 2023;1436:69-85. doi: 10.1007/5584_2022_758. Adv Exp Med Biol. 2023. PMID: 36689167
Cited by
-
Autophagy as a Therapeutic Target in Breast Tumors: The Cancer stem cell perspective.Autophagy Rep. 2024 Jun 24;3(1):2358648. doi: 10.1080/27694127.2024.2358648. eCollection 2024 Dec 31. Autophagy Rep. 2024. PMID: 39006309 Free PMC article.
-
Exploring Importance and Regulation of Autophagy in Cancer Stem Cells and Stem Cell-Based Therapies.Cells. 2024 Jun 1;13(11):958. doi: 10.3390/cells13110958. Cells. 2024. PMID: 38891090 Free PMC article. Review.
-
Tumor dormancy and relapse: understanding the molecular mechanisms of cancer recurrence.Mil Med Res. 2025 Feb 11;12(1):7. doi: 10.1186/s40779-025-00595-2. Mil Med Res. 2025. PMID: 39934876 Free PMC article. Review.
-
Autophagy as a potential therapeutic target in regulating improper cellular proliferation.Front Pharmacol. 2025 May 15;16:1579183. doi: 10.3389/fphar.2025.1579183. eCollection 2025. Front Pharmacol. 2025. PMID: 40444035 Free PMC article. Review.
-
A comprehensive analysis of the relationship between inflammasomes and autophagy in human tumors: Recent developments.J Cell Commun Signal. 2025 Jul 16;19(3):e70035. doi: 10.1002/ccs3.70035. eCollection 2025 Sep. J Cell Commun Signal. 2025. PMID: 40671967 Free PMC article. Review.
References
-
- Tran K.B., Lang J.J., Compton K., Xu R., Acheson A.R., Henrikson H.J., Kocarnik J.M., Penberthy L., Aali A., Abbas Q., et al. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400:563–591. doi: 10.1016/S0140-6736(22)01438-6. - DOI - PMC - PubMed
-
- Nowell P. La evolución clonal de las poblaciones de células tumorales. Ciencia. 1976;194:23–28.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

