A Possible Therapeutic Application of the Selective Inhibitor of Urate Transporter 1, Dotinurad, for Metabolic Syndrome, Chronic Kidney Disease, and Cardiovascular Disease
- PMID: 38474414
- PMCID: PMC10931163
- DOI: 10.3390/cells13050450
A Possible Therapeutic Application of the Selective Inhibitor of Urate Transporter 1, Dotinurad, for Metabolic Syndrome, Chronic Kidney Disease, and Cardiovascular Disease
Abstract
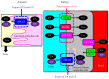
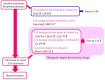
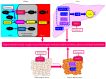
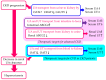
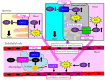
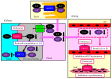
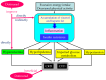
The reabsorption of uric acid (UA) is mainly mediated by urate transporter 1 (URAT1) and glucose transporter 9 (GLUT9) in the kidneys. Dotinurad inhibits URAT1 but does not inhibit other UA transporters, such as GLUT9, ATP-binding cassette transporter G2 (ABCG2), and organic anion transporter 1/3 (OAT1/3). We found that dotinurad ameliorated the metabolic parameters and renal function in hyperuricemic patients. We consider the significance of the highly selective inhibition of URAT1 by dotinurad for metabolic syndrome, chronic kidney disease (CKD), and cardiovascular disease (CVD). The selective inhibition of URAT1 by dotinurad increases urinary UA in the proximal tubules, and this un-reabsorbed UA may compete with urinary glucose for GLUT9, reducing glucose reabsorption. The inhibition by dotinurad of UA entry via URAT1 into the liver and adipose tissues increased energy expenditure and decreased lipid synthesis and inflammation in rats. Such effects may improve metabolic parameters. CKD patients accumulate uremic toxins, including indoxyl sulfate (IS), in the body. ABCG2 regulates the renal and intestinal excretion of IS, which strongly affects CKD. OAT1/3 inhibitors suppress IS uptake into the kidneys, thereby increasing plasma IS, which produces oxidative stress and induces vascular endothelial dysfunction in CKD patients. The highly selective inhibition of URAT1 by dotinurad may be beneficial for metabolic syndrome, CKD, and CVD.
Keywords: ATP-binding cassette transporter G2; chronic kidney disease; dotinurad; hyperuricemia; organic anion transporter1/3; urate transporter 1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The Long-Term Effects of the Selective Inhibitor of Urate Transporter 1, Dotinurad, on Metabolic Parameters and Renal Function in Japanese Patients With Asymptomatic Hyperuricemia.J Clin Med Res. 2025 Jun 9;17(6):320-333. doi: 10.14740/jocmr6250. eCollection 2025 Jun. J Clin Med Res. 2025. PMID: 40641858 Free PMC article.
-
Pharmacological Evaluation of Dotinurad, a Selective Urate Reabsorption Inhibitor.J Pharmacol Exp Ther. 2019 Oct;371(1):162-170. doi: 10.1124/jpet.119.259341. Epub 2019 Aug 1. J Pharmacol Exp Ther. 2019. PMID: 31371478
-
Potentiation of the Uricosuric Effect of Dotinurad by Trans-Inhibition of the Uric Acid Reabsorptive Transporter 1.Drug Metab Dispos. 2023 Nov;51(11):1527-1535. doi: 10.1124/dmd.123.001412. Epub 2023 Aug 29. Drug Metab Dispos. 2023. PMID: 37643882
-
Dotinurad: a novel selective urate reabsorption inhibitor as a future therapeutic option for hyperuricemia.Clin Exp Nephrol. 2020 Mar;24(Suppl 1):1-5. doi: 10.1007/s10157-019-01811-9. Epub 2019 Nov 21. Clin Exp Nephrol. 2020. PMID: 31754883 Free PMC article. Review.
-
Dotinurad: a novel selective urate reabsorption inhibitor for the treatment of hyperuricemia and gout.Expert Opin Pharmacother. 2021 Aug;22(11):1397-1406. doi: 10.1080/14656566.2021.1918102. Epub 2021 Apr 30. Expert Opin Pharmacother. 2021. PMID: 33926357 Review.
Cited by
-
Lychee Peel Extract Ameliorates Hyperuricemia by Regulating Uric Acid Production and Excretion in Mice.Curr Issues Mol Biol. 2025 Jan 25;47(2):76. doi: 10.3390/cimb47020076. Curr Issues Mol Biol. 2025. PMID: 39996797 Free PMC article.
-
The Long-Term Effects of the Selective Inhibitor of Urate Transporter 1, Dotinurad, on Metabolic Parameters and Renal Function in Japanese Patients With Asymptomatic Hyperuricemia.J Clin Med Res. 2025 Jun 9;17(6):320-333. doi: 10.14740/jocmr6250. eCollection 2025 Jun. J Clin Med Res. 2025. PMID: 40641858 Free PMC article.
-
Cathepsin B-dependent glycolysis contributes to reduced renal uric acid excretion in hyperuricemia.Commun Biol. 2025 Jun 2;8(1):845. doi: 10.1038/s42003-025-08303-5. Commun Biol. 2025. PMID: 40457023 Free PMC article.
-
Identifying reliable obesity indices for hyperuricemia among middle-aged and elderly populations: a longitudinal study.Lipids Health Dis. 2024 Sep 26;23(1):305. doi: 10.1186/s12944-024-02296-6. Lipids Health Dis. 2024. PMID: 39327579 Free PMC article.
-
Novel Potential Probiotics from Chinese Baijiu Fermentation Grains: Dual Action of Lactiplantibacillus plantarum LTJ1/LTJ48 in Uric Acid Reduction and Gut Microbiota Restoration for Hyperuricemia Therapy in Mice.Nutrients. 2025 Jun 24;17(13):2097. doi: 10.3390/nu17132097. Nutrients. 2025. PMID: 40647202 Free PMC article.
References
-
- Yanai H., Adachi H., Hakoshima M., Katsuyama H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021;22:9221. doi: 10.3390/ijms22179221. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

