Nicotinamide Riboside Augments Human Macrophage Migration via SIRT3-Mediated Prostaglandin E2 Signaling
- PMID: 38474420
- PMCID: PMC10931126
- DOI: 10.3390/cells13050455
Nicotinamide Riboside Augments Human Macrophage Migration via SIRT3-Mediated Prostaglandin E2 Signaling
Abstract
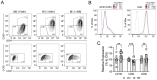
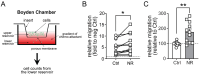
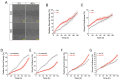
NAD+ boosting via nicotinamide riboside (NR) confers anti-inflammatory effects. However, its underlying mechanisms and therapeutic potential remain incompletely defined. Here, we showed that NR increased the expression of CC-chemokine receptor 7 (CCR7) in human M1 macrophages by flow cytometric analysis of cell surface receptors. Consequently, chemokine ligand 19 (CCL19, ligand for CCR7)-induced macrophage migration was enhanced following NR administration. Metabolomics analysis revealed that prostaglandin E2 (PGE2) was increased by NR in human monocytes and in human serum following in vivo NR supplementation. Furthermore, NR-mediated upregulation of macrophage migration through CCL19/CCR7 was dependent on PGE2 synthesis. We also demonstrated that NR upregulated PGE2 synthesis through SIRT3-dependent post-transcriptional regulation of cyclooxygenase 2 (COX-2). The NR/SIRT3/migration axis was further validated using the scratch-test model where NR and SIRT3 promoted more robust migration across a uniformly disrupted macrophage monolayer. Thus, NR-mediated metabolic regulation of macrophage migration and wound healing may have therapeutic potential for the topical management of chronic wound healing.
Keywords: NAD+ boosting; SIRT3; chemotaxis; macrophage migration; nicotinamide riboside; prostaglandin E2.
Conflict of interest statement
Dr. Sack’s laboratory studies mechanisms of action of nicotinamide riboside (NR) on immunomodulation in human inflammatory disease. His laboratory receives no direct financial support or compensation but does acquire NR and matching placebo from Chromadex Inc. through Cooperative and Development Research Agreements (CRADA) Material Transfer Agreements.
Figures



Similar articles
-
Nicotinamide Riboside-Driven Modulation of SIRT3/mtROS/JNK Signaling Pathways Alleviates Myocardial Ischemia-Reperfusion Injury.Int J Med Sci. 2024 Aug 12;21(11):2139-2148. doi: 10.7150/ijms.97530. eCollection 2024. Int J Med Sci. 2024. PMID: 39239543 Free PMC article.
-
Nicotinamide riboside attenuates myocardial ischemia-reperfusion injury via regulating SIRT3/SOD2 signaling pathway.Biomed Pharmacother. 2024 Jun;175:116689. doi: 10.1016/j.biopha.2024.116689. Epub 2024 May 3. Biomed Pharmacother. 2024. PMID: 38703508
-
Activation of SIRT3 by the NAD⁺ precursor nicotinamide riboside protects from noise-induced hearing loss.Cell Metab. 2014 Dec 2;20(6):1059-68. doi: 10.1016/j.cmet.2014.11.003. Cell Metab. 2014. PMID: 25470550 Free PMC article.
-
Emerging Role of Nicotinamide Riboside in Health and Diseases.Nutrients. 2022 Sep 20;14(19):3889. doi: 10.3390/nu14193889. Nutrients. 2022. PMID: 36235542 Free PMC article. Review.
-
NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR.Cell Metab. 2018 Mar 6;27(3):513-528. doi: 10.1016/j.cmet.2017.11.002. Epub 2017 Dec 14. Cell Metab. 2018. PMID: 29249689 Free PMC article. Review.
References
-
- Youm Y.H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., D’Agostino D., Planavsky N., Lupfer C., Kanneganti T.D., et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21:263–269. doi: 10.1038/nm.3804. - DOI - PMC - PubMed
-
- Johnson J.B., Summer W., Cutler R.G., Martin B., Hyun D.-H., Dixit V.D., Pearson M., Nassar M., Tellejohan R., Maudsley S., et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. - DOI - PMC - PubMed
-
- Fraser D.A., Thoen J., Djoseland O., Forre O., Kjeldsen-Kragh J. Serum levels of interleukin-6 and dehydroepiandrosterone sulphate in response to either fasting or a ketogenic diet in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 2000;18:357–362. - PubMed
-
- Choi I.Y., Piccio L., Childress P., Bollman B., Ghosh A., Brandhorst S., Suarez J., Michalsen A., Cross A.H., Morgan T.E., et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016;15:2136–2146. doi: 10.1016/j.celrep.2016.05.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

