Chlorine-Induced Toxicity on Murine Cornea: Exploring the Potential Therapeutic Role of Antioxidants
- PMID: 38474422
- PMCID: PMC10930774
- DOI: 10.3390/cells13050458
Chlorine-Induced Toxicity on Murine Cornea: Exploring the Potential Therapeutic Role of Antioxidants
Abstract
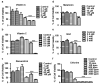
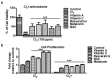
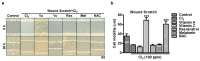
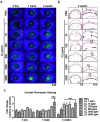
Chlorine (Cl2) exposure poses a significant risk to ocular health, with the cornea being particularly susceptible to its corrosive effects. Antioxidants, known for their ability to neutralize reactive oxygen species (ROS) and alleviate oxidative stress, were explored as potential therapeutic agents to counteract chlorine-induced damage. In vitro experiments using human corneal epithelial cells showed decreased cell viability by chlorine-induced ROS production, which was reversed by antioxidant incubation. The mitochondrial membrane potential decreased due to both low and high doses of Cl2 exposure; however, it was recovered through antioxidants. The wound scratch assay showed that antioxidants mitigated impaired wound healing after Cl2 exposure. In vivo and ex vivo, after Cl2 exposure, increased corneal fluorescein staining indicates damaged corneal epithelial and stromal layers of mice cornea. Likewise, Cl2 exposure in human ex vivo corneas led to corneal injury characterized by epithelial fluorescein staining and epithelial erosion. However, antioxidants protected Cl2-induced damage. These results highlight the effects of Cl2 on corneal cells using in vitro, ex vivo, and in vivo models while also underscoring the potential of antioxidants, such as vitamin A, vitamin C, resveratrol, and melatonin, as protective agents against acute chlorine toxicity-induced corneal injury. Further investigation is needed to confirm the antioxidants' capacity to alleviate oxidative stress and enhance the corneal healing process.
Keywords: JC-1; ROS; antioxidants; chlorine; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Mitochondria-targeted antioxidant SKQ1 protects cornea from oxidative damage induced by ultraviolet irradiation and mechanical injury.BMC Ophthalmol. 2018 Dec 27;18(1):336. doi: 10.1186/s12886-018-0996-7. BMC Ophthalmol. 2018. PMID: 30587174 Free PMC article.
-
N-acetyl cysteine mitigates lung damage and inflammation after chlorine exposure in vivo and ex vivo.Toxicol Appl Pharmacol. 2023 Nov 15;479:116714. doi: 10.1016/j.taap.2023.116714. Epub 2023 Oct 10. Toxicol Appl Pharmacol. 2023. PMID: 37820773
-
An Immunohistochemical Study of the Increase in Antioxidant Capacity of Corneal Epithelial Cells by Molecular Hydrogen, Leading to the Suppression of Alkali-Induced Oxidative Stress.Oxid Med Cell Longev. 2020 Jun 21;2020:7435260. doi: 10.1155/2020/7435260. eCollection 2020. Oxid Med Cell Longev. 2020. Retraction in: Oxid Med Cell Longev. 2022 May 9;2022:9831406. doi: 10.1155/2022/9831406. PMID: 32655773 Free PMC article. Retracted.
-
Chlorine-induced cardiopulmonary injury.Ann N Y Acad Sci. 2016 Jun;1374(1):159-67. doi: 10.1111/nyas.13091. Epub 2016 Jun 15. Ann N Y Acad Sci. 2016. PMID: 27303906 Free PMC article. Review.
-
The role of corneal crystallins in the cellular defense mechanisms against oxidative stress.Semin Cell Dev Biol. 2008 Apr;19(2):100-12. doi: 10.1016/j.semcdb.2007.10.004. Epub 2007 Oct 10. Semin Cell Dev Biol. 2008. PMID: 18077195 Review.
Cited by
-
Endoplasmic Reticulum Stress Induces ROS Production and Activates NLRP3 Inflammasome Via the PERK-CHOP Signaling Pathway in Dry Eye Disease.Invest Ophthalmol Vis Sci. 2024 Dec 2;65(14):34. doi: 10.1167/iovs.65.14.34. Invest Ophthalmol Vis Sci. 2024. PMID: 39699913 Free PMC article.
-
Oxidative Stress and Antioxidant Strategies: Relationships and Cellular Pathways for Human Health.Cells. 2024 Nov 11;13(22):1871. doi: 10.3390/cells13221871. Cells. 2024. PMID: 39594619 Free PMC article.
References
-
- Andley U.P., Rhim J.S., Chylack L.T., Jr., Fleming T.P. Propagation and immortalization of human lens epithelial cells in culture. Investig. Ophthalmol. Vis. Sci. 1994;35:3094–3102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

