Synthesis and Anti-Inflammatory Activity of Ferulic Acid-Sesquiterpene Lactone Hybrids
- PMID: 38474447
- PMCID: PMC10933920
- DOI: 10.3390/molecules29050936
Synthesis and Anti-Inflammatory Activity of Ferulic Acid-Sesquiterpene Lactone Hybrids
Abstract
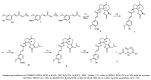
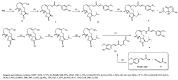
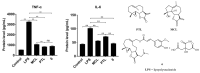
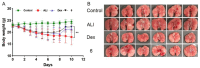
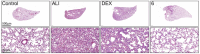
Acute lung injury (ALI) is a respiratory failure disease associated with high mortality rates in patients. The primary pathological damage is attributed to the excessive release of pro-inflammatory mediators in pulmonary tissue. However, specific therapy for ALI has not been developed. In this study, a series of novel ferulic acid-parthenolide (FA-PTL) and ferulic acid-micheliolide (FA-MCL) hybrid derivatives were designed, synthesized, and evaluated for their anti-inflammatory activities in vitro. Compounds 2, 4, and 6 showed pronounced anti-inflammatory activity against LPS-induced expression of pro-inflammatory cytokines in vitro. Importantly, compound 6 displayed good water solubility, and treatment of mice with compound 6 (10 mg/kg) significantly prevented weight loss and ameliorated inflammatory cell infiltration and edema in lung tissue, as well as improving the alveolar structure. These results suggest that compound 6 (((1aR,7aS,8R,10aS,10bS,E)-8-((dimethylamino)methyl)-1a-methyl-9-oxo-1a,2,3,6,7,7a,8,9,10a,10b-decahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-5-yl)methyl (E)-3-(4-hydroxy-3-methoxyphenyl)acrylate 2-hydroxypropane-1,2,3-tricarboxylate) might be considered as a lead compound for further evaluation as a potential anti-ALI agent.
Keywords: acute lung injury; anti-inflammatory activity; ferulic acid; micheliolide; parthenolide.
Conflict of interest statement
Authors Yanwei Zhang, Xiaoguang Huo, Shiqi Bao, Zhuo Shen and Xuemei Zhang were employed by the company Accendatech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Sesquiterpene lactones and cancer: new insight into antitumor and anti-inflammatory effects of parthenolide-derived Dimethylaminomicheliolide and Micheliolide.Front Pharmacol. 2025 Feb 20;16:1551115. doi: 10.3389/fphar.2025.1551115. eCollection 2025. Front Pharmacol. 2025. PMID: 40051564 Free PMC article. Review.
-
Protective effect of sesquiterpene lactone parthenolide on LPS-induced acute lung injury.Arch Pharm Res. 2016 Dec;39(12):1716-1725. doi: 10.1007/s12272-016-0716-x. Epub 2016 Oct 18. Arch Pharm Res. 2016. PMID: 27757770
-
Dehydrocostus Lactone Suppresses LPS-induced Acute Lung Injury and Macrophage Activation through NF-κB Signaling Pathway Mediated by p38 MAPK and Akt.Molecules. 2019 Apr 17;24(8):1510. doi: 10.3390/molecules24081510. Molecules. 2019. PMID: 30999647 Free PMC article.
-
Local administration of p-coumaric acid decreases lipopolysaccharide-induced acute lung injury in mice: In vitro and in silico studies.Eur J Pharmacol. 2021 Apr 15;897:173929. doi: 10.1016/j.ejphar.2021.173929. Epub 2021 Feb 6. Eur J Pharmacol. 2021. PMID: 33561444
-
Sesquiterpene Lactones and Cancer: New Insight into Antitumor and Anti-inflammatory Effects of Parthenolide-Derived Dimethylaminomicheliolide and Micheliolide.Comput Math Methods Med. 2022 Jul 18;2022:3744837. doi: 10.1155/2022/3744837. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Jul 12;2023:9856528. doi: 10.1155/2023/9856528. PMID: 35898475 Free PMC article. Retracted. Review.
Cited by
-
Design, synthesis, antifungal, and antibacterial evaluation of ferulic acid derivatives bearing amide moiety.Mol Divers. 2024 Dec 27. doi: 10.1007/s11030-024-11076-4. Online ahead of print. Mol Divers. 2024. PMID: 39729179
-
Sesquiterpene lactones and cancer: new insight into antitumor and anti-inflammatory effects of parthenolide-derived Dimethylaminomicheliolide and Micheliolide.Front Pharmacol. 2025 Feb 20;16:1551115. doi: 10.3389/fphar.2025.1551115. eCollection 2025. Front Pharmacol. 2025. PMID: 40051564 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

