Exploring Novel Antidepressants Targeting G Protein-Coupled Receptors and Key Membrane Receptors Based on Molecular Structures
- PMID: 38474476
- PMCID: PMC10934989
- DOI: 10.3390/molecules29050964
Exploring Novel Antidepressants Targeting G Protein-Coupled Receptors and Key Membrane Receptors Based on Molecular Structures
Abstract
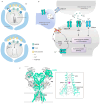
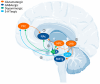
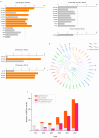
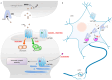
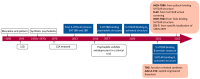
Major Depressive Disorder (MDD) is a complex mental disorder that involves alterations in signal transmission across multiple scales and structural abnormalities. The development of effective antidepressants (ADs) has been hindered by the dominance of monoamine hypothesis, resulting in slow progress. Traditional ADs have undesirable traits like delayed onset of action, limited efficacy, and severe side effects. Recently, two categories of fast-acting antidepressant compounds have surfaced, dissociative anesthetics S-ketamine and its metabolites, as well as psychedelics such as lysergic acid diethylamide (LSD). This has led to structural research and drug development of the receptors that they target. This review provides breakthroughs and achievements in the structure of depression-related receptors and novel ADs based on these. Cryo-electron microscopy (cryo-EM) has enabled researchers to identify the structures of membrane receptors, including the N-methyl-D-aspartate receptor (NMDAR) and the 5-hydroxytryptamine 2A (5-HT2A) receptor. These high-resolution structures can be used for the development of novel ADs using virtual drug screening (VDS). Moreover, the unique antidepressant effects of 5-HT1A receptors in various brain regions, and the pivotal roles of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and tyrosine kinase receptor 2 (TrkB) in regulating synaptic plasticity, emphasize their potential as therapeutic targets. Using structural information, a series of highly selective ADs were designed based on the different role of receptors in MDD. These molecules have the favorable characteristics of rapid onset and low adverse drug reactions. This review offers researchers guidance and a methodological framework for the structure-based design of ADs.
Keywords: G protein-coupled receptors; cryo-electron microscopy; major depressive disorder; novel antidepressants; structure-based drug design; virtual drug screening.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Fornaro M., Anastasia A., Novello S., Fusco A., Pariano R., De Berardis D., Solmi M., Veronese N., Stubbs B., Vieta E.J.P.r. The emergence of loss of efficacy during antidepressant drug treatment for major depressive disorder: An integrative review of evidence, mechanisms, and clinical implications. Pharmacol. Res. 2019;139:494–502. doi: 10.1016/j.phrs.2018.10.025. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82272348, 82270013, 81870016 and 82300016/National Natural Science Foundation of China
- 2021QN02Y720/High Talent Project of Guangdong Province
- 2023A1515030195, 2022A1515011223 and 2022A1515010525/Natural Science Foundation of Guangdong Province
- 2021KTSCX038/Characteristic Innovation Project of Universities in Guangdong Province
- 2022ZDZX 2021/Key Project of Universities in Guangdong Province
- 2022KCXTD010/Innovation Team Project of Universities in Guangdong Province
- 20211800905072, 20211800904782 and 20211800905542/Science and Technology Project of Dongguan
- 4SG22209G/Doctoral Initial Funding of Guangdong Medical University
- 4SG23290G, 4SG21229GDGFY01, 4SG22259G, 4SG23030G and 4SG23077G/Discipline Construction Project of Guangdong Medical University
- 4SG23143G, 4SG22098G and 1JG21027/In Vitro Diagnostics Industry College Project
- 2021SLABFN10/Open Research Fund of Songshan Lake Materials Laboratory
- 2022kfkt01/Open Research Fund for Key Laboratory of Tropical Disease Control Sun Yat-sen University, Ministry of Education
- GDMUD2022001/Youth Research Projects of Guangdong Medical University
LinkOut - more resources
Full Text Sources

