Detection of 26 Drugs of Abuse and Metabolites in Quantitative Dried Blood Spots by Liquid Chromatography-Mass Spectrometry
- PMID: 38474487
- PMCID: PMC10934168
- DOI: 10.3390/molecules29050975
Detection of 26 Drugs of Abuse and Metabolites in Quantitative Dried Blood Spots by Liquid Chromatography-Mass Spectrometry
Abstract
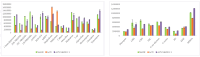
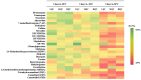
A method was developed for the determination of 26 drugs of abuse from different classes, including illicit drugs in quantitative dried blood spots (qDBSs), with the aim to provide a convenient method for drug testing by using only 10 μL of capillary blood. A satisfactory limit of quantification (LOQ) of 2.5 ng/mL for 9 of the compounds and 5 ng/mL for 17 of the compounds and a limit of detection (LOD) of 0.75 ng/mL for 9 of the compounds and 1.5 ng/mL for 17 of the compounds were achieved for all analytes. Reversed-phase liquid chromatography was applied on a C18 column coupled to MS, providing selective detections with both +ESI and -ESI modes. Extraction from the qDBS was performed using AcN-MeOH, 1:1 (v/v), with recovery ranging from 84.6% to 106%, while no significant effect of the hematocrit was observed. The studied drugs of abuse were found to be stable over five days under three different storage conditions (at ambient temperature 21 °C, at -20 °C, and at 35 °C), thus offering a highly attractive approach for drug screening by minimally invasive sampling for individuals that could find application in forensic toxicology analysis.
Keywords: LC–MS/MS analysis; blood micro sampling; drug screening; drugs of abuse; quantitative dried blood spot.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Quantitative determination by UHPLC-MS/MS of 18 common drugs of abuse and metabolites, including THC and OH-THC, in volumetric dried blood spots: a sustainable method with minimally invasive sampling.J Chromatogr B Analyt Technol Biomed Life Sci. 2024 Oct 15;1247:124337. doi: 10.1016/j.jchromb.2024.124337. Epub 2024 Oct 9. J Chromatogr B Analyt Technol Biomed Life Sci. 2024. PMID: 39401474
-
Liquid-liquid extraction solvent selection for comparing illegal drugs in whole blood and dried blood spot with LC-MS-MS.J Anal Toxicol. 2025 Jan 20;49(1):26-35. doi: 10.1093/jat/bkae081. J Anal Toxicol. 2025. PMID: 39366924 Free PMC article.
-
Quantification of cocaine and cocaine metabolites in dried blood spots from a controlled administration study using liquid chromatography-tandem mass spectrometry.Drug Test Anal. 2019 May;11(5):709-720. doi: 10.1002/dta.2537. Epub 2018 Dec 13. Drug Test Anal. 2019. PMID: 30379417
-
Sensitive screening of abused drugs in dried blood samples using ultra-high-performance liquid chromatography-ion booster-quadrupole time-of-flight mass spectrometry.J Chromatogr A. 2017 Mar 31;1491:57-66. doi: 10.1016/j.chroma.2017.02.037. Epub 2017 Feb 21. J Chromatogr A. 2017. PMID: 28238428
-
The application of fully automated dried blood spot analysis for liquid chromatography-tandem mass spectrometry using the CAMAG DBS-MS 500 autosampler.Clin Biochem. 2020 Aug;82:33-39. doi: 10.1016/j.clinbiochem.2020.02.007. Epub 2020 Feb 19. Clin Biochem. 2020. PMID: 32087137 Review.
References
-
- Hannon W.H., Therrell B.L. In: Overview of the History and Applications of Dried Blood Samples. Li W., Lee M.S., editors. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2014. pp. 1–15.
-
- Eshghi A., Pistawka A.J., Liu J., Chen M., Sinclair N.J.T., Hardie D.B., Elliott M., Chen L., Newman R., Mohammed Y., et al. Concentration Determination of >200 Proteins in Dried Blood Spots for Biomarker Discovery and Validation. Mol. Cell. Proteom. 2020;19:540–553. doi: 10.1074/mcp.TIR119.001820. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

